
|
 |
|
{Page 59}
School of Chemical Engineering and Materials Science, University of Oklahoma, Norman, Oklahoma
The optimum operating temperature and pH have been determined for a catalyst prepared by the insolubilization of a hepatic microsomal mixed-function oxidase on alumina. An optimum oxygen concentration has also been determined for the catalyst bound on glass beads. Temperature is a more significant parameter than pH in calculating potential catalyst yield. An optimum pH of 8.1, temperature of 27 C, and an oxygen concentration of 45% have been observed with respect to alumina- and glass bead-bound oxidase. The immobilized enzyme catalyzes the NADPH- and oxygen-dependent N-oxidation of a variety of tertiary and other N-substituted amines. The function of this reaction system is to produce enzymically new drugs and related metabolites that are difficult or impossible to synthesize by standard chemical techniques.
| Introduction | Materials | Methods | Results and Discussion | References | Table of Contents | Home |
Enzyme insolubilization has been employed with success and is described in a number of excellent reviews (1—3). A mixed-function microsomal flavoprotein oxidase (MFMF) has been reported to retain its specificity and exhibit a remarkably increased thermal stability upon insolubilization on nylon tubing, Sepharose particles, glass beads, and other dense matrices (4, 5). The insolubilized oxidase catalyzes the N-oxidation of tertiary amines and other N-substituted amines (6, 7) and hydrazines (8), and the S-oxidation of thioureylenes (9).
The purpose of optimizing this enzyme-catalyzed reaction system is to obtain products which are difficult or impossible to synthesize by direct chemical means. In compounds that contain more than one readily oxidized group the enzyme catalyzes the oxidation at only one position. For example, only the side chain of phenothiazine drugs such as chlorpromazine is oxidized:
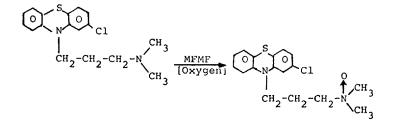
There is no evidence that the enzyme catalyzes the oxidation of the ring nitrogen or sulfur atoms. On the other hand, direct chemical oxidation usually produces a variety of products which are difficult to purify.
The synthesis of amine oxide derivatives of a variety of drugs in sufficient quantities for animal testing is possible with reactors using the insolubilized mixed-function oxidase as the catalyst. This article describes the effects of pH and temperature on catalyst activity and life and defines the optimum conditions for maximum product formation as a function of unit quantities of the alumina-mounted oxidase.
| Introduction | Materials | Methods | Results and Discussion | References | Table of Contents | Home |
The mixed-function amine oxidase, isolated from pig liver microsomes, was the generous gift of Dr. D. M. Ziegler of the Clayton Foundation Biochemical Institute at the University of Texas at Austin. G6PD, G6P, NADP+, and NADPH were obtained from Sigma Chemical Company. N,N-Dimethylaniline was purchased from Eastman Chemical Company and purified by gas-liquid chromatography. Alumina was obtained from the Aluminum Company of America.
| Introduction | Materials | Methods | Results and Discussion | References | Table of Contents | Home |
The oxidase was immobilized on alumina by a modification of the method of R. W. Coughlin (10). This involves the activation of alumina by treatment with titanium tetrachloride and subsequent covalent linkage of the enzyme to the matrix surface. The immobilized enzyme was stored at 5 C in 0.025M potassium phosphate buffer, pH 7.4, when not in use.
The activity of alumina- and bead-bound oxidase was obtained by measuring the substrate-dependent increase in oxygen consumption in a liquid-sealed, temperature-controlled 1.9-ml micro-assay reactor con-
{Page 60}
nected to a Clark-type oxygen electrode. The signal from the electrode was recorded with a Heath (Model EC-2058 strip chart recorder. The reaction media contained 0.2 mM NADP+, 10 mM glucose 6-phosphate, sufficient glucose 6-phosphate dehydrogenase to reduce no less than 1 µmole NADP+ /min/ml at 25 C, and 0.02M potassium phosphate — 0.02M sodium pyrophosphate buffer adjusted to maintain the pH indicated in the legends to the figures. The pH listed was measured directly by microprobe in the reaction medium at the specified reaction temperature. The reaction medium was stirred with a magnetic stirring bar that generated sufficient turbulence that mass diffusion to the exterior of the catalyst particle was not rate limiting (4, 5). Oxygen sensitivity studies were conducted at room pressure and 37 C. Oxygen gas concentration was varied by dilution with nitrogen gas. A few minutes were allowed for temperature equilibration and the reaction was started by the injection of substrate to a final concentration of 1 mM. The oxygen concentration in the liquid was monitored continuously to obtain the substrate-dependent rate of oxygen consumption. Catalyst activities per gram were measured for alumina matrix by methods described previously (4, 5) for a glass bead matrix.
The stoichiometry of the reaction is known and the accuracy of the calculated rate of substrate oxidation is dependent mainly on the precision of oxygen concentration measurements. With a properly calibrated electrode, oxygen concentrations may readily be determined with an error of ±2%.
| Introduction | Materials | Methods | Results and Discussion | References | Table of Contents | Home |
The optimizations were conducted with the model compound N,N-dimethylaniline (DMA) as substrate. DMA is easily obtainable in pure form at a low price. The oxidation of DMA by the soluble enzyme has been studied in great detail (6, 7). The concentration of this substrate required to half-saturate the purified oxidase is in the µM range, well below its upper limits of solubility in water. Further, the pKa of DMA is below 5 and changes in the concentration of the free amine as a function of pH are marginal over the pH range studied. In addition, DMA does not inhibit the oxidase even at relatively high substrate concentrations.
Figure 1 indicates the rate of reaction as a function of pH. The maximum ranges between pH 8.2 and 8.4. This value compares well with the pH optimum observed with soluble enzyme and glass bead-bound oxidase (5). The decrease in activity observed at pH 8.5 also is observed with the free enzyme and glass bead-mounted oxidase. Yield maximization of product per unit mass of catalyst as a function of pH requires a plot of catalyst half-life with respect to pH (Figure 2). This plot represents roughly a straight line on a semi-log scale. The gain in reaction rate obtained by operating at the pH range of 8.2-8.4 would be offset by the high rate of enzyme decomposition in that range. Figure 3 illustrates this variation of total product/catalyst yield as a func-
{Page 61}
tion of pH. This is obtained by assuming a first order decomposition of the alumina-supported catalyst and integrating over infinite time.
A similar maximization procedure was conducted with respect to temperature. Catalyst activity and half-life, as a function of temperature, were determined and are illustrated in Figures 4 and 5 respectively. The dotted line in Figure 4 represents the fast decomposition of the catalyst at high temperatures. The half-life at 55 C is approximately 2 minutes. The half-life plot in Figure 5 shows that the enzyme is essentially stable at lower temperatures. The yield maximization as a function of temperature indicates as shown in Figure 6 that the optimal operating temperature is approximately 27 C.
A modified maximization approach had been utilized in obtaining the optimum operating oxygen concentration. Figure 7 shows the reaction rate as a function of oxygen concentration for glass bead catalyst. Very low activities were obtained at low oxygen concentrations. However, significant catalyst degradation and consequently lower reaction rates occur at high oxygen
{Page 62}
concentrations. This is shown by the dotted lines. As shown in Figure 8, catalyst is most stable at lower oxygen concentrations. Product yield as a function of oxygen concentration is shown in Figure 9. An optimal operating oxygen concentration of 45% has been obtained.
The establishment of operating parameters with respect to pH, temperature, and oxygen concentration is only a first step toward the design of a reactor to produce drug oxides in gram quantities. Further, while fluidized-bed reactors are routinely being used in this laboratory, other reactor configurations remain to be considered. We recognize the fact that the optimum pH, temperature, and oxygen concentrations are not indeed mutually independent. For example, the pH optimum was determined at 37 C, and is not necessarily the same at 27 C. A more rigorous optimization technique is planned involving measurements under actual reaction conditions in a re-circulation reactor. However, the "optimums" obtained as described in this paper have, indeed, led to greatly increased product yields.
| Introduction | Materials | Methods | Results and Discussion | References | Table of Contents | Home |
1. K. MOSBACH, Sci. Am. 224, No. 3: 26-33 (1971).
2. O. R. ZABORSKY, Immobilized Enzymes, CRC Press, Cleveland, Ohio, 1973.
3. R. D. FALB, Biotechnol. Bioeng. Symp. No. 3: 177-84 (1972).
4. S. S. SOFER, D. M. ZIEGLER, and R. P. POPOVICH, Biochem. Biophys. Res. Commun. 57: 183-9 (1974).
5. S. S. SOFER, D. M. ZIEGLER, and R. P. POPOVICH, Biotech. Bioeng. 17: 107-17 (1975).
6. D. M. ZIEGLER, C. H. MITCHELL, and D. JOLLOW, in J. R. Gillett, A. H. Conney, G. J. Cosmides, R. W. Estabrook, J. R. Fouts and G. J. Mannering (eds.), Microsomes and Drug Oxidations, Academic Press, New York, 1969, p. 173.
7. D. M. ZIEGLER and C. H. MITCHELL, Arch. Biochem. Biophys. 150: 116-25 (1972).
8. R. A. PROUGH, Arch. Biochem. Biophys. 158: 442-4 (1973).
{Page 63}
9. L. L. POULSEN, R. M. HYSLOP, and D. M. ZIEGLER, Biochem. Pharmacol., in press.
10. R. W. COUGHLIN, et al., Biochem. Biophys. Res. Commun. 57: 1054-62 (1964).
11. J. R. FORD, A. H. LAMBERT, W. COHEN, and R. P. CHAMBERS, Biotechnol. Bioeng. Symp. No. 3: 267-84 (1972).
12. PIERCE REVIEWS, published by Pierce Chemical Company, Rockford, Illinois, U.S.A., April 1973.
13. E. A. OSINOWO, Initial Design of a Reactor for an Enzyme-Catalyzed Drug Oxidation Reaction, Master's thesis, The University of Oklahoma, 1976.
| Introduction | Materials | Methods | Results and Discussion | References | Table of Contents | Home |