
|
 |
|
{Page 93}
Oklahoma Water Resources Board, Oklahoma City, Oklahoma
| Top of Page | References | Table of Contents | Home |
The impact of brine discharge and regular releases from the Foss Demineralization Plant (FDP) and Foss Reservoir (FR), respectively, on the chemical characteristics of 27 miles of the Washita River below Foss Reservoir in Custer County, Oklahoma, has been determined (1). Indices that describe the community structure of benthic macroinvertebrates aid water quality evaluations by portraying the present habitat conditions as well as those in the recent past (2, 3, 4, 5). Therefore, in conjunction with the chemical evaluation of the Washita River below Foss Reservoir, a biological evaluation was planned utilizing community diversity indices.
A disadvantage to a biological water quality evaluation is the large amount of time needed to identify the organisms collected, which reduces the timeliness of the results. To prevent this loss of timeliness, partial results — the benthic community structure of the Washita River from February, 1973 to August, 1973 prior to the 1975 commencement of FDP and FR discharges — are presented here.
Four samples were taken at each of 10 sites (Fig. 1) every month except for Feb-
{Page 94}
ruary and August for the above period. The petite ponar grab was chosen as the sampling device because of substrate and stream characteristics of the Washita River and the comparable efficiency of the ponar with other dredge samplers (4). Practical considerations discouraged the use of artificial substrates. Grab samples were taken near each bank and two near midstream to obtain representative stream substrate conditions. A transect angled upstream was followed at each site to avoid disturbing the substrate prior to sampling. Each grab was sieved in-stream with a wash frame containing a 30-mesh brass screen, transferred to glass containers (1 to 0.5 1) and preserved with 95% ethanol containing 0.2 g/l of Rose Bengal stain to facilitate sorting.
Level of identification varied depending on difficulty of each different group and remained consistent for each lowest practical taxon. Empty mollusc shells were treated as stream inhabitants and all molluscs were identified according to shell characters. Many taxonomic studies were used, but Ward and Whipple (6), Pennak (7), and Mason (8) were the main references.
Equations 1 and 2 were used to calculate the community diversity and redundancy indices.
Equation 1. Brillouin (9),
Community Diversity
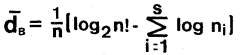
| where: | ni = number of individuals per taxon n = total number of individuals s = total number of taxa in sample |
Equation 2. Margalef (10),
Redundancy
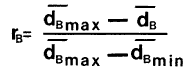
where: ![]()
and ![]()
with n and s the same as in Equation 1.
The Brillouin equation was used because it is preferred when small sample sizes, n < 100, are encountered (11). The equation proposed by Wilhm and Dorris (2) is an approximation and should be used only when n > 100 (4).
The indices representing the community structure for March, 1973, through July, 1973, were analyzed with two-way ANOVAS without replication (12) to detect differences between the 10 sites and five months. The indices for February and August, 1973, were not included with these analyses because of incomplete sampling. One subsample each from April - site 3, June - site 1, and August - site 2 was discarded owing to preservative evaporation.
{Page 95}
A total of 13,811 benthic macroinvertebrates representing 108 taxa were collected during this period. The majority of individuals (Fig. 2) and taxa (Fig. 3) were dipterans, specifically 42 genera of Chironomidae with Psectrocladius predominating (31.8%). This species is tolerant to a wide range of environmental conditions (13). Gyraulus and Sphaerium were the most abundant molluscs. Musculium was treated as synonymous with Sphaerium. The following groups were represented by one specimen each: Hydrozoa, Hirudinea, Crustacea, and Neuroptera. The Oligochaeta were represented by 1700 specimens. Only three Plecopteran were collected.
Most of the individuals not included in the community structure indices were 610 chironomid pupae, 190 organisms separated only for major group recognition, and four unrecognizable organisms. Further investigation is under way to verify rare taxa found; at present, only an incomplete list of taxa collected is available (Table 1).
The null hypothesis was rejected for the community structure indices comparing months (dB ¯at P < 0.001, and 0.05 > P > 0.01 for rB ). A decrease in mean diversities occurred from the largest one in March to the lowest diversity representing June and then a slight increase occurred in July (Table 2). This decrease was expected considering the normal emergence of the mature insect fauna as summer approached. The largest range of diversities for the five months analyzed occurred in April and ranged from 0.91 to 3.97 at sites 3 and 5,
{Page 96}
respectively. This fluctuation of diversities might be partially explained by a spring insect emergence. The mean redundancies for March through July ranged from 0.32 to 0.51 (Table 2) illustrating a slight dominance among taxa, specifically Gyraulus, Psectrocladius, oligochaetes, Physa, ceratopogons and Cryptochironomus.
Although there was a gradual increase in total dissolved solids (TDS) from site 1 downstream to site 10 (1), no significant differences were found between sites for dB or rB. The effect of TDS, mainly sulfates and chlorides, on benthic communities might be small and require a more thorough sampling to detect a difference.
Diversity indices greater than 3 have been found in good-quality water and values of 2 and 1 in progressively worse-quality water (2). Utilizing this system to evaluate water quality, the Washita River was found to exhibit clean water in March and was moderately polluted in the Spring (Table 2). Categorizing the Washita River as moderately polluted might be inappropriate, because the term pollution legally refers to an indirect or direct water quality degradation influenced by man (15), whereas the high mineral content found in the Washita River during this period (1) is principally derived from natural gypsum formations.
These water quality generalizations based only on benthic community indices can be misleading if not qualified. On account of procedural differences in sampling and mathematical computation, diversity comparisons should be performed with caution, if at all. The species diversity in the North Fork of the Red River near Headrick, Oklahoma, was reported to be 2.82 on June 30, 1976 (14), and the mean diversity for 10 sites on the Washita River was 1.94 in June, 1973; yet the TDS of 1717.9 mg/ l, mainly chlorides, in the North Fork of the Red River was higher than the Washita River's TDS of 360 mg/ l, primarily sulfates. Therefore, the effect of chlorides on the benthic macroinvertebrates could exceed the sulfates impact, partially explaining the lower diversity value for the Washita River. Comparatively, the Washita River's water quality in reference to TDS is average for the Upper Red River Basin (15).
Many aspects need to be considered before classifying water quality, not just benthic diversity indices. Considering nutrients, minerals and metal concentrations, the Oklahoma State Health Department ranked the Washita River water quality below most of the major tributaries in the Red River drainage basin (14). In view of the high mineral content and low community diversities, the Washita River had poor water quality from April through July, 1973, but qualified for eight beneficial uses including irrigation (15).
Robert Kellogg, limnologist, Oklahoma State Department of Health, identified most of the organisms and supervised the field collections. The project was originally proposed by D. A. Haws, W. D. Motsenbocker and R. W. Gasper. We thank these individuals along with the many other Water Board staff members who were instrumental in this research. This investigation was funded by the Oklahoma Water Resources Board.
| Top of Page | References | Table of Contents | Home |
1. M. P. MADDEN and W. K. MORRIS, Proc. Okla. Acad. Sci., 58: 88-92 (1978).
2. J. L. WILHM and T. C. DORRIS, Bio. Sci. 18: 477-481 (1968).
3. J. L. WILHM, J. Water Poll. Contr. Fed. 39: 1673-1683 (1967).
4. C. I. WEBER, Macroinvertebrates in Biological and Laboratory Methods for Measuring the
{Page 97}
Quality of Surface Waters ad Effluents, Nat. Environ. Res. Center, Environ. Prot. Agency, Cincinnati, Ohio, 1973.
5. K. V. SLACK, R. C. AVERETT, P. E. GREESON, and R. C. LIPSCOMB, Methods for collection and analysis of aquatic biological and microbiological samples, in Techniques of Water-Resources Investigations of the United States Geological Survey, Dept. of Interior, 1973, pp. 24-25.
6. W. T. EDMONDSON, Freshwater Biology, John Wiley & Sons, New York, 1959.
7. R. W. PENNAK, Fresh-water Invertebrates of the United States, The Ronald Press, New York, 1953.
8. W. T. MASON, An Introduction to the Identification of Chironomid Larvae, Anal. Qual. Contr. Lab., Nat. Environ. Res. Center, Environ. Prot. Agency, Cincinnati, Ohio, 1973.
9. L. BRILLOUIN, Science and Information Theory, Academic Press, New York, 1962, pp. 1-10.
10. D. R. MARGALEF, General Systems 3: 36-71 (1958).
11. R. D. HARKINS and R. E. AUSTIN, J. Water Poll. Contr. Fed 45: 1606-1611 (1973).
12. R. R. SOKAL and F. J. ROHLF, Biometry, W. R. Freeman and Co., San Francisco, 1969.
13. W. T. MASON, JR., P. A. LEWIS, and J. B. ANDERSON, Macroinvertebrate Collections and Water Quality Monitoring in the Ohio River Basin 1963-1967, Office of Technical Programs, Ohio Basin Region and Analytical Quality Control Laboratory, Water Quality Office, Environ. Prot. Agency, Cincinnati, Ohio, 1973.
14. OKLAHOMA STATE DEPT. OF HEALTH, Surface Water Quality Assessment for Oklahoma Water Year 1976 (305b Report), Oklahoma City, Oklahoma, 1977.
15. OKLAHOMA WATER RESOURCES BOARD, Oklahoma's Water Quality Standards 1976 (Publication 79), Oklahoma City, Okla., 1977.
| Top of Page | References | Table of Contents | Home |