
|
 |
|
{Page 70}
Department of Physical Sciences, Southeastern Oklahoma State University, Durant, OK 74701
This report describes electric discharge-induced reactions which occur under heterogeneous conditions in mixtures of methane, ammonia, and water. These may be related to processes taking place in the visible aerosols of the planet Jupiter since conditions there are comparable (e.g., a similar gas mixture is subjected to lightning discharges). The reaction system examined in this report produces liquid mixtures of simple intermediates, and these in turn form more complex products, ultimately insoluble solids; thus the initial products are amenable to studies by NMR. Previous simulations by other authors have been limited because their conditions led directly to glassy, insoluble products.
| Introduction | Methods | Results | Discussion | References | Table of Contents | Home |
Mixtures of methane, ammonia, and water yield complex products when subjected to electric discharges (1), photochemical stress (2), ionizing radiation (3), or intense shock waves (4). The products of these suprathermal processes are typically brown, insoluble residues which contain an abundance of inorganic and organic cyanides and amines. The residues decompose to a variety of organic compounds upon acid hydrolysis (5).
Similar brown colorations are observed in the atmosphere aerosols of the planets Jupiter and Saturn, and since methane, ammonia, and (to some extent) water are present, one may guess that closely related chemical reactions could be taking place. Sagan and Khare (6) suggest the term "tholin" (a derivative from the Greek language meaning roughly, "sky mud") to describe the brown residues. The latter authors also interpret the infrared absorption features of interstellar clouds as evidence for tholin-like substances on the dust grains.
This paper reports a preliminary NMR-oriented kinetic study of a tholin-forming reaction. The notable feature of the system described here is its progression from relatively simple precursors, via soluble intermediates, to an insoluble end-product. The latter substance was found to be closely comparable to an insoluble material observed by the above-cited authors (6).
| Introduction | Methods | Results | Discussion | References | Table of Contents | Home |
Reaction Apparatus
The essential features of the reactor are shown in Fig. 1. A gas mixture was prepared by bubbling methane through concentrated ammonium hydroxide at a flow rate of 25 ml / min (1 atm) and 25 C. This mixture was routed through a 5000-V AC, 15-mA electric discharge (between platinum electrodes). The discharge was maintained at pressures between 4 and 8 mm Hg where a yellow-green glow was observed. Effluent from the discharge gap then passed through a trap immersed in liquid N2 (77 K) in which intermediate compounds accumulated. In some experiments the trap was fitted with an air-tight siphon (not shown in Fig. 1) for the purpose of transferring liquid samples to NMR cuvettes. Transfers of this type were done after venting the low-boiling gases from the trap since the NMR measurements were obtained at temperatures at or above ambient. In no case was air permitted to come into contact with the sample.
Materials
Highest-purity methane was obtained from Matheson, Inc. Concentrated ammonium hydroxide was of analytical reagent grade purity. Reference substances were obtained from Aldrich Chemical Company.
{Page 71}
NMR Spectra
1H-NMR spectra were obtained by means of a Varian EM-360 spectrometer (60 MHz). The modifications of this instrument have been described in detail (7,8) and include fast scan/correlation detection. Unassigned resonances were compared with known substances by direct additions of the latter to the reaction mixtures. All shifts are relative to external tetramethylsilane.
Infrared Spectra
Spectra were obtained from samples dried over P2O5 and prepared as pressed KBr pellets. Infrared absorption recordings were obtained on a Beckman Acculab-10 spectrophotometer.
Spectrophotometry at Visible Wavelengths
A computer-interfaced McKee-Pedersen model MP-1018 UV/visible spectrophotometer was used to obtain quantitative absorbance measurements in the 400 - 700 nm range. Details of the interface between the instrument's RCA 931A photomultiplier and an Altair microcomputer have been reported (8). Its usual cuvette was replaced with an aperture stop and centering collar for the purpose of obtaining absorbances from sealed 5-mm NMR cuvettes; thus both NMR spectra and optical absorbances could be obtained from the same sample.
Activation Energy of the Pigment-Forming Reaction
Data for an Arrhenius plot were obtained as follows: Cold-trap residues were warmed to 40 C, transferred to NMR cuvettes (using the air-tight siphon), and held at 40 C for 300 min, which insured that the pigment-forming process had been initiated. The cuvettes were then transferred to water baths at different temperatures, and the absorbance change at 600 nm during a 15-min time interval was recorded. The small size of the NMR cuvette permitted rapid thermal equilibration.
| Introduction | Methods | Results | Discussion | References | Table of Contents | Home |
A disadvantage of the experimental arrangement shown in Figure 1 is its tendency to cause a change in the composition of the reacting gas mixture, e.g., NH3 will be depleted as the reaction progresses. To compensate for this, the bubbler entrainment flask contained 1.5 liters of concentrated NH4OH. In this work the flow reaction was allowed to continue for two hours.
The Pigment-Forming Reaction
On warming the trap contents to ambient and venting off volatile components, a liquid residue consisting chiefly of concentrated NH4OH remained. The latter was straw-colored at first but began to
{Page 72}
darken. As shown in Fig. 2, the rate of pigment formation was not greatest immediately after warming the specimen to 40 C. Instead, the rate began to increase after a delay of approximately three hours. Absorbance increases beyond this period are clearly thermochemical in origin, as shown by the Arrhenius plot of Fig. 3. The reaction can be virtually stopped if the cuvette is kept in an ice bath, and visible light appears to have no accelerating affect on the chilled mixture.
As shown in Fig. 4, 1H-NMR absorptions (signals 8,9,10, and 11) appear in the aromatic/olefinic region as the reacting mixture darkens. Also, these NMR signals appeared on a time scale which was closely comparable to that of Fig. 2 (Figs. 2 and 4b were obtained under identical conditions). The structure or structures responsible for signals 8-11 are not known. Even though Figures 2 and 4b show a similar time dependence, it does not necessarily follow that the NMR absorptions and the optical spectrum are due to the same chemical species. For example, the chromophore might have a large molar extinction coefficient at visible wavelengths, in which case the amount required to produce the observed absorbance at 600 nm might be virtually undetectable by NMR.
In view of the kind of elements present in the reacting mixture (carbon, hydrogen, nitrogen and to a lesser extent, oxygen) a chromophore based on conjugated unsaturated groups is plausible. The initial reaction products apparently include HCN and organic cyanides (vide infra) and the latter substances may polymerize (9) in various ways:
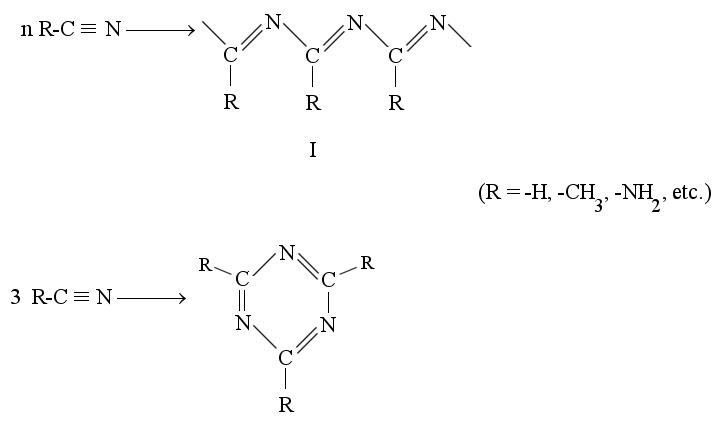
The -C º N stretching absorption (2330 cm-1) is prominent in the initial reactor effluent but diminishes with time, as would be expected of the reactions shown above. Mixed polymerization involving both organic and inorganic cyanides would lead to relatively complex structures, and 1H-NMR chemical shifts similar to those of signals 8-11 may be expected for structural types I and II when the R-group is hydrogen. The tholin formed from this mixture absorbs strongly in the 1630-1690 cm-1 band, where >C=N— stretching is expected. Extended
{Page 73}
polymers of type I may also absorb at visible wavelengths. It is emphasized that these possibilities are only speculative since the available evidence is insufficient for unequivocal identification of the chemical species producing NMR and optical spectra.
Absorptions which increase after a delay (signals 13 and 14) also appear in the aliphatic region of the NMR spectrum, as shown in Figs. 5a and 5b. Figure 6 is an attempt to present the net spectral features associated with the pigmentation process. Bars beneath this spectrum indicate regions where absorptions may have been obscured by interfering resonances.
There is no reason to believe that this spectrum represents a single compound. The brown-colored material was observed to contain cationic, anionic, and neutral components when subjected to electrophoresis on Sepraphore III. A densitometer scan of a representative electrophoreogram is shown in Fig. 7. The breadth of the migrating bands of Fig. 7 is indicative of distributions rather than single entities, showing that the mixture is indeed quite complex.
Precipitation begins to occur after about three days if the specimen is maintained at 30 C. Dilution of the sample with water appears to hasten this irreversible precipitation. The infrared spectrum of the insoluble material is shown in Fig. 8 in comparison with the spectrum of a "spark tholin" (6). These appear to be similar, although the strong absorptions at 3300, 2200, 1650, and 800 cm-1 could be interpreted to reflect a substantial water content in both specimens. The visible absorption spectrum of our soluble intermediate shows a bathochromic shift of 100 nm relative to that found by Sagan and Khare for their tholin (6) as shown in Fig. 9. A dif-
{Page 74}
ference of this magnitude is understandable if the soluble tholin precursor encountered in our work contains amine substituents on the chromophore moiety. Alternatively, if both spectra originate in the same type of chromophore, e.g., a polymerized cyanide, then the observed shift could be due to a differing extent of conjugation.
Precursor Substances
1H-NMR spectra obtained revealed principally three singlet resonances (signals 2,3, and 6) which decreased with time, as shown in Fig. 10. The decrease in these resonances was found to be quantitatively comparable to the increase in the resonances of Fig. 6. General features of the NMR spectrum are collected in Table I.
NMR signal 2 has been identified as due to methylamine while the smaller signal 4 proved to arise from acetonitrile. Signal 6 occurs slightly downfield from a resoance of aminoacetonitrile, but the latter coincides with minor signal 7. Signals 3 and 6 are present in a 2:3 ratio and could be due to N-methylaminoacetonitrile. The initial mixture may also include ammonium cyanide and diaminomaleonitrile (10). It is interesting to note that various mixtures of these substances in NH4OH form the familiar brown color, and further simulation of this system is currently in progress.
The NMR spectra were conspicuously silent in the aldehyde region, even at high gain, ruling out Strecker-type reactions. Also, significant amounts of absorption were not found at the chemical shift regions associated with glycine, dimethylamine, trimethylamine, and propionitrile. Consistent with the work of other investigators, acid hydrolysis led to ninhydrin-positive compounds. One of the latter substances appeard to be glycine (based on paper chromatography). Acid hydrolysis of aminoacetonitrile should produce glycine, and it has been shown that aminacetonitrile is present in the reaction mixture.
{Page 75}
| Introduction | Methods | Results | Discussion | References | Table of Contents | Home |
It is notable that two of the precursors of tholin (acetonitrile and methylamine) are substances found in interstellar molecular clouds (6). The presence of amino nitriles in sparked mixtures of methane and ammonia was established by Ponnamperuma et al. (11). However, these authors' proposed mechanism of pyrimidine formation from these substances cannot be quantitatively significant in the system considered here; a dearth of olefinic/aromatic resonances in the initial mixture does not favor much participation of electron impact-induced oxidation in the spark gap. Rather, the formation of conjugated structures appears to be associated with a thermochemical reaction of methylamine, amino nitriles, and other cyanides, possibly involving polymerization of the cyanide groups.
Electric discharge reactions occurring at reduced pressures produce substantial quantities of free radicals. Possible sequences leading to the characterized species are as follows, where activation steps are marked (a) :
CH4¾¾®a CH4* ¾¾® CH3• + H•
NH3¾¾®a NH3* ¾¾® NH2• + H•
NH2• + CH3• ¾¾® CH3NH2¾¾¾
CH3NH2 ¾¾® HCN + 2H2 (i.e., pyrolysis)stepwise
HCN ¾¾®a HCN* ¾¾® H• + •CN
CH3• + •CN ¾¾® CH3CN¾¾¾
CH3CN ¾¾®a CH3CN* ¾¾® •CH2CN + H•
NH2• + CH2CN ¾¾® NH2CH2CN¾¾¾¾¾
The free radical mechanisms suggested here are highly speculative. Ion/molecule reactions which lead to similar products (3) should occur only at gas pressures much lower than those encountered in this work and are probably insignificant factors in this system.
It is emphasized that the conditions of the reaction described here are in no way expected to simulate accurately those in the atmospheres of the giant planets. For example, a more realistic mixture would be richer in elemental hydrogen. Similarities between the infrared spectra (Fig. 8) of the two types of tholins may also be superficial, i.e., most complex mixtures would be expected to show broad envelopes. The major advantage associated with this system is its suitability for high resolution NMR spectroscopy, e.g., other methods produce only intractable solids. If it proves possible to establish a close chemical relationship between tholins prepared by this method and others, then NMR analysis should pro-
{Page 76}
[Page 76 consist entirely of Table 1.]
{Page 77}
vide insight into their structural features. Raman and resonance Raman scattering spectra should also be applicable to this system.
This research was supported partially by NIH Grant 2 506 RR 08003-10. Barbara Wright and Sharon Dunning typed the manuscript.
| Introduction | Methods | Results | Discussion | References | Table of Contents | Home |
1. B. N. KHARE and C. SAGAN, Astrophys. Space Sci. 65: 309-312 (1979).
2. B. N. KHARE, C. SAGAN, E. L. BANDURSKI, and B. NAGY, Science 199: 1199-1201 (1978).
3. M. S. B. MUNSON and F. H. FIELD, J. Am. Chem. Soc. 87: 4242-4247 (1976).
4. A. BAR-NUN, N. BAR-NUN, S. H. BAUER, and C. SAGAN, Science 168: 470-472 (1970).
5. S. L. MILLER, J. Am. Chem. Soc. 77: 2351-2361 (1955).
6. C. SAGAN and B. N. KHARE, Nature 277: 102-107 (1979).
7. J. R. WRIGHT, Rev. Sci. Instr. 49: 1288-1292 (1978).
8. C. R. BEAVERS, S. E. GEORGE, J. L. ROBINSON, and J. R. WRIGHT, in: P. LYKOS, Ed., Personal Computers in Chemistry, John Wiley, New York, 1981, pp. 58-84.
9. J. J. CHRISTIANSEN, H. D. JOHNSON, and R. M. IZZATT, J. Chem. Soc., A, 454-461 (1970).
10. S. YUASA and M. ISHIGAMI, Origins of Life 6: 75-81 (1975).
11. M. A. CHADHA, P. M. MOLTON, and C. PONNAMPERUMA, Origins of Life 6: 127-136 (1975).