
|
 |
|
{Page 67}
Jimmie Pigg, Mark S. Coleman, Jay Wright, and Robert Gibbs
State Environmental Laboratory, Oklahoma Department of Environmental Quality Oklahoma City, Oklahoma 73117-1295.
Keith Gido and Roger R. Lemmons
Zoology Department, University of Oklahoma, Norman, Oklahoma 73070.
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
Between 1976 and 1996 the fish of the Deep Fork River were sampled at three sites. A total of 163,358 specimens from 36 species was taken. To determine if changes had occurred since 1976 as a result of modifications in the aquatic environment, the fish communities at each of the three sampling stations were compared. Three periods during which well defined environmental changes that resulted in changes in the quality and quantity of the water were identified. Alterations of the fish community parameters were most pronounced during periods of poor water quality; dam construction; and long-term, regulated flows from an impoundment. The changing patterns of the community characteristics during this 21 yr period are summarized with special emphasis on their connection with certain changes in the aquatic habitat of the river. Significant temporal trends occurred in the number of species (P=0.01); mean number of Lepomis megalotis, Gambusia affinis, Cyprinella lutrensis; number of specimens for collections; mean biotic index (P<0.01); and mean number of Centrarchidae. Significant (P<0.05) spatial trends occurred in number of species; mean number of Gambusia affinis and Cyprinella lutrensis, mean number of specimens for collection, and mean number of centrarchidae The Interaction between site and time was significant (P=0.001) in mean number of L. megalotis (P=0.001), mean biotic index (P=0.001), and mean number of centrarchids (P=0.001). ©1998 Oklahoma Academy of Science
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
The introduction of wastewater and other domestic, industrial and agricultural runoff into a body of water can result in the displacement and/or destruction of the fish community. Such disruptions can be of either short or long duration, depending upon the nature of the imposed stress and the recovery process (1). Historically, there have been few, intensive investigations of the ichthyofauna, of small, urban Oklahoma rivers that are fed solely by storm runoff waters and treated sewage effluent. There is little long-term information available on the chemistry and biology of these affected lotic environments. Most studies have been limited in scope, for example fish population studies or water quality investigations in a specific stream segment.
The Deep Fork River receives mainly urban storm runoff waters from highly populated areas. It heads in northwestern and northeastern Oklahoma City and southeastern Edmond and flows northeast into Lake Arcadia. Studies of the fishes of the Deep Fork River by personnel from the Oklahoma State Department of Health (OSDH) between 1976 and 1980 indicate that the Deep Fork River had few fish species in the upper reaches. The fish community was limited because of the release of poorly treated effluents from domestic sewage treatment plants (STP) in Oklahoma City and Edmond. These effluents adversely affected the biological and chemical water quality in the river (unpublished data OSDH).
Soon after the establishment of the OSDH, now the Oklahoma Department of En-
{Page 68}
vironmental Quality (ODEQ), biomonitoring program in 1976, three study sites were established on the Deep Fork River. To delineate the temporal and spatial distribution of fish at selected sites, collections were taken by staff members two or three times yearly by seining, gill netting, and electrofishing. This paper documents long-term species diversity and distribution of fish in Deep Fork River and examines the possibility that changes in fish populations since 1976 are related to changes in the environment. We describe the changes in the fish communities as the river underwent changes due to the impact of urban runoff storm waters; discharge of poorly treated sewage effluents into the river; construction of a dam; filling of Lake Arcadia; and, since 1980, regulated flows from the lake. The ecological impacts of changes in regulated streams are well documented, but very little is known for small urban rivers in Oklahoma.
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
Deep Fork River in central Oklahoma drains an area of 78,453 km2 . The headwaters are in Britton, in northeastern Oklahoma City. The river flows through a long, narrow basin eastward for 370 km to its confluence with the North Canadian River in Lake Eufaula. The basin is elongated with an average width of 40.2 km (Fig. 1).
There are two reservoirs on the Deep Fork River, Lake Arcadia and Lake Eufaula. Lake Arcadia is at T04N R02W S36 in Oklahoma County, in the metropolitan areas of Oklahoma City and Edmond (Fig. 1). Construction started in October 1980, and the reservoir became operational in November 1986. Other small lakes in the Deep Fork drainage include Belle Isle, Northeast, Aluma, Hiwassee, Clark, Okemah City, Stroud, Okmulgee, and Henryetta.
The basin slopes an average of 0.7 m/km eastward from a maximum elevation of 412 m above mean sea level in Oklahoma County, to about 153 m at Lake Eufaula. Most of the area surrounding the river is characterized by low to moderate slopes. The area has fast runoff and erosion is high. The river has a gradient of 9.1 m/km in the extreme upper reaches of the river. Channel capacity varies from 56.6 m3/s at Luther to about 113 m3/s at its mouth. [Editor's note: Because the cited flow rates, below, vary so widely, they are given in m3/s or L/s, as appropriate; conversion is straight forward, since 1 m3/s = 1000 L/s.] The small channel capacity of the Deep Fork has produced a long history of flooding. Record flooding has produced water levels 3.4 m above bank full, and the river flooded twice a year, on average (2). The river length from Oklahoma City to the Creek County line was channelized in the late 1920s for flood control.
The land use patterns in the Deep Fork River drainage include open agricultural areas, such as field crops, pecan orchards, and pasture lands; urban areas, such as parks, Oklahoma City Zoo, race track, and urban renewal areas; and forested, red sandstone hills. Approximately 40% of the upper basin lies within Oklahoma County and Oklahoma City. The lower Deep Fork River Basin is predominantly rural, with agriculture as the major land use. The only urbanized area is the headwaters region in Oklahoma City and Edmond.
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
Fish were collected by shoreline seining covering 200 m, using a nylon, "Common Sense" minnow seine, a 3.0 ×1.5-m heavily-leaded minnow seine. To ensure standard sampling, we attempted to make 20 seine hauls of 10 m each. Efforts were limited to approximately 1 hr at each site. Each sampling event at a site used the same starting and finishing points. Collections of fish began in 1976, and since 1981 collections were made two or three times yearly and have continue unchanged today (1997). Large fish were collected by electrofishing or gill netting at Sites 1 and 3 and were retained for analysis of toxic substances in the tissue. Sport fish or large non-sport fish were identified in the field, counted, measured for total length (TL), and weighed; they were released or wrapped in foil and placed on ice.
Seining samples were preserved in 10% formalin in the field and taken to the laboratory for processing: identification to species; measuring TL range and total weight of each
{Page 69}
species; examination for disease, tumors, and deformed bodies. After soaking and washing in water, all specimens were transferred to [45% 2-propanol-55% H2O], and deposited at the Oklahoma Museum of Natural History (UOMNH) or Oklahoma State University, Department of Zoology Collections of Vertebrates (OSUS). Fish records from UOMNH and OSUS were examined for comparison.
Observations and measurement were made of the physical habitat at all sites during each visit. Color slides of upstream and downstream views were made to provide a permanent habitat record for each site. These data are maintained in field notebooks at ODEQ and eventually will be given to UOMNH.
Long-term surveys of water, sediment, and fish tissue for the analysis of toxic constituents have been carried out by OSDH/ODEQ since 1976. The United States Geological Survey (USGS) first sampled water quality in 1907 and then again from 1969 to 1989 from Site 1 west of Arcadia (2). Longterm USGS water quality records for sites upstream from Lake Arcadia are rare. USGS water quality records from the headwaters of the Deep Fork River are available for the following sites: (a) Portland Avenue in Oklahoma City, 1979-1980, and (b) Eastern Avenue in Oklahoma City, 1973-1974; (c) a site near Witcher, 1959 and 1973; and (d) a site at Witcher, 1960-1963 and 1975-1976. Other USGS water quality sites downstream from Lake Arcadia are at Warwick (near Chandler), Stroud, Beggs, Dewar, and Pierce. Daily mean stream discharge values are also available for the Lake Arcadia Site 1, 1969-1989 (2).
During each visit water samples were collected. Dissolved oxygen (DO), water temperature, and secchi disk values were measured in the field. Additional analyses, for pH, turbidity, and specific conductance, were performed on the water samples in the ODEQ State Environmental Laboratory in Oklahoma City.
Species diversity was calculated from both numerical and biomass data with the Shanon-Weaver Index (3):
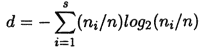
|
where ni is the number or the biomass for each ith species, n is the total number of individuals or total biomass, and s is the total number of species. Diversity computed for each collection from each sampling site was used to calculate the diversity over the sampling period.
Indices of similarity (S) were computed for comparison between stations and between collections. In this study the similarity index was used for comparison of fish community among stations and years. The index of community similarity was used to examine similarities in fish collections between years. These values range from zero, no similarity -- no species are in common, to 1.00 -- all taxa are shared by two locations or collections, with <0.40 considered low and >0.74 being high (4).
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
Site selection was based on the location and construction of Lake Arcadia. Three long-term sites were originally established downstream from the proposed Lake Arcadia. Site 1, located southeast of Arcadia, was in a zone that was impacted by storm runoff waters, treated sewage effluent, dam construction, long periods of regulated flows, and discharges from the lake. Site 2, located north of Luther, was in the transition zone of the river; however, this site received 110 L/s of treated wastewater from the Edmond Sewage STP via Coffee Creek. Site 3, southwest of Wellston, was selected because it is in the recovery zone of the river (Fig. 1). Other factors considered in site selection were accessibility by roads; seasonal persistence of flows; historical data on water quality, water quantity data, and fish collections.
We identified three periods with well defined environmental factors that produced changes in both water quality and quantity. These periods were identified as (a) 1976-1980, which we called the Poor Water Quality Period (PWQ); (b) 1981-1985, the Construction Period (CNF) in which the flow regime was controlled by rain events; and (c) 1986-1995, Regulated Flow Period (RFP), the post-construction period with regulated flows in which the flow regime was controlled by releases of water from the lake.
{Page 70}
During the PWQ period, the river received daily between 657 and 1314 L/s of poorly treated sewage effluent from the Northside Oklahoma City STP and the Edmond Spring Creek STP. During low flow conditions the base flow of the stream was sewage. The STP discharges were between 453 and 566 L/s. During wet periods, flows were a combination of storm runoff from urban streets and the Oklahoma City Zoo and poorly treated sewage. Flow patterns during the PWQ period were highly variable with a maximum flow 405 m3/s. Storm events usually produced flooding. Site 1 had 42 d/yr with elevated flows greater than 28 m3/s. A minimum flow of 127 L/s was fairly stable because of continuous daily discharges from the two STPs. Only once during this period was the flow less than 28 L/s. During this period, two or three flood events occurred each year, each lasting from 3 to 5 d.
The number of violations of the Water Quality Standards of Oklahoma is evidence of the poor water quality during the period. During the PWQ period violations were recorded as percent of samples collected which exhibited the indicated violation: (a) 7%, low dissolved oxygen (DO) <5.0 mg/L); (b) 4%, high water temperature >32 °C; (c) 2%, low pH <6.5); (d) 10%, high nitrite >10 ppm); (e) 1%, high chlorides >327 ppm), (f) 1%, high sulfates >201 ppm): (g) 15%, high turbidity >50 units); and heavy metals: such as 5%, arsenic; 3%, total cadmium; 7%, chromium; 10%, copper; 3%, lead; and 3%, nickel (Table 1) (unpublished OSDH data).
During the CNF period, 1981-85, the major environmental impacts were the construction of Lake Arcadia and the removal of the Oklahoma City Northside STP and the Spring Creek Edmond STP effluents from the river. Partial, regulated flows during the construction stage produced a maximum flow of 368 m3/s of water. Flows depended entirely upon storm runoff events. The removal of the STPs also produced a slightly smaller mean daily flow of 1,846 L/s. Five low-flow, <28 L/s days were recorded, and one 0.0 L/s day occurred. Elevated flows, >2.8 m3/s occurred an average of 20 d/yr.
During the RFP period, 1986-1996, after construction of Lake Arcadia, there was a long period of regulated lake discharges and long periods of continuously high discharge with flushing-out flows. Regulation of lake releases has resulted in longer periods of low-flow or no-flow conditions. By acting as a nutrient and toxin trap, the lake has improved the water quality in Deep Fork River. We observed substantial changes in the substrata downstream from the lake due to the scouring out of loose, downstream sediments. This caused the loss of pool habitats. The alternating flow patterns produced a maximum flow of 42 m3/s maximum flows were much smaller in earlier years. A minimum flow of 0.09 L/s and many days without measurable flow were recorded. The mean daily discharge of 2.1 m3/s was higher during this period because flushing-out periods alternated with continuous higher-flows periods than was typical in earlier years. There were 157 d/yr of low-flow, <263 L/s, conditions. There was a mean of 122 d/yr of continuous low flow (<28 L/s). During the periods of regulated flow, a mean of 66 d/yr of elevated flows (>2.8 m3/s occurred. The periods of evaluated flows usually consisted of 6-26 d of continuous flows, >2.8 m3/s. Extreme high-flows periods, >28 m3/s varied in length from 4 to 6 d each year (Table 2).
During the 36 y before impoundment, under natural conditions the flow exceeded 57 m3/s 67 times. Under regulated conditions, post-impoundment, flow has never exceeded 57 m3/s. Table 3 summaries the indicator variables used to describe the differences in numbers of individuals collected due to type of water discharge practice (a) sewage discharges (PWQ) vs. partially regulated discharge (CNF), (b) sewage discharge (PWQ) vs. regulated discharge (RFP), and (c) partially regulated discharge (CNF) vs. regulated discharge (RFP).
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
Station 1 is 1.6 km southwest of Arcadia on Post Road in Oklahoma County (T14N R01W S29). The site is a USGS water quality station (U07342350) that was established in October
{Page 71}
1969 and discontinued in 1989. The site is 1.9 km downstream from Lake Arcadia Dam and 2.6 km upstream from the confluence with Coffee Creek. It has a drainage area of 168.9 km2.
The mean discharge for the 17 yr prior to regulation by the Arcadia Dam was 1,877 L/s. The mean since regulation, 1986-1992, has been higher, 2,086 L/s. The maximum discharge for this site was 405 m3/s on 2 November 1974, while the minimum discharge was 0.9 L/s on 10 July 1988. Since regulation there have been long periods of no measurable flow (Table 2).
At Site 1 there are high (20-30 m), steep, almost vertical, red clay banks. The vertical banks have little ground cover and are highly eroded. There is a large amount of bank slumping throughout the area. The banks have a small band of hardwood trees and thick Johnson grass on both sides. The banks at the bridge have large rocks or rip-rap. Waste concrete from the old bridge was dumped into the stream forming a long rocky riffle, resulting in a fairly stable channel.
The aquatic habitat consisted of a long (100-120 m), narrow (3-5 m), and shallow (0.3-0.6 m) pool. During low-flow periods, this pool was covered (80-90%) with a thick floating mat of green algae. The waste concrete formed a long (30-40 m), very narrow (1-2 m) channel of fast >1.0 m/s) rapids at high-flow periods and small (0.3-2.0 m), slow <0.2 m/s), shallow <0.3 m) riffles during low-flow periods. During low-flow periods, long, thick mats, 40-50%, of filamentous green algae were attached to most rocks. Downstream, the riffle enters a long (70-80 m), narrow (10-15 m) pool, which is fairly deep (1.0-1.5 m) with shallow edges and beds of green filamentous algae. The pool ends with small (3.0 m), fast (0.4-0.6 m/s), shallow (4.0-12.0 cm) riffles and a small backwater pool.
Station 2 was first located 0.8 km west of Luther Road on U.S. Hwy 66 near Luther, Oklahoma County (T14N R01E S28). Due to high, steep vertical banks (30-40 m), and bank slumping, we were forced to move the site 400 m downstream to the bridge on Luther Road, 0.8 km north of Luther. This site is 14.5 km northeast of Site 1 (Fig. 1).
At Site 2, the width between banks was 101 m. The channel was 32 m wide with banks that are high (20-25 m), but not as steep as at Site 1. The banks have more cover and are more stable than banks at Sites 1 and 3. These banks are covered with thick growths of grass and weeds, which lessens bank erosion. At the bridge, there is a layer of large limestone boulders that form rip-rap. These boulders, with concrete waste from the old bridge, have provided protection for the banks. A old oil line was at this site and hydrocarbon odors were noted several times. The river between Sites 1 and 2 drains mainly rural farming areas.
The upper section of Site 2 consists of narrow (3 m), deep (0.8 m) runs, and wide (6 m), shallow (0.2 m) riffles. The channel forms a fast >1.0 m/s), deep (1.0 m) run along the north bank. The center of the channel and the east bank are a very shallow <0.5 m), long (20-25 m), slow <0.2 m/s) riffle with some divided channels. There are several small backwater inlets and occasionally isolated pools. At the lower section of the site a deep (2.0-2.5 m) instream pool is found. In the main channel, old tires, wood debris, rocks, and concrete waste provided some instream cover. During low-flow periods, thick mats of filamentous green algae were anchored on most structures in the water.
Station 3 is located at the northwest corner of Wellston on the road to Ingram in Lincoln County (T14N R02E S11) at the mouth of Captain Creek. It is located 11.2 km northeast of Site 2. Site 3 was selected to be in the recovery zone of the river. This originally was the least impacted site, but during our study it underwent major habitat changes. Before 1986, this site had the most diverse aquatic habitat of all three sites.
At Site 3, the banks full width was 95 m, and the stream width was 46 m with high (20-30 m), steep, bare banks of highly eroded, reddish, fine grain, sandy loam. The banks were covered with dense grasses and sunflowers. Each flood event caused additional bank slumping and filled the pools with sediment. Additional sediment was supplied by Captain Creek.
{Page 72}
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
An environmental factor that may have influenced the fish communities of the Deep Fork River is nutrient loading from municipal STP with potential daily discharges of 657-1,314 L/s. The river between northwest Oklahoma City and Wellston, Site 3, was rated at one time by OSDH as one of the most nutrient-enriched streams in Oklahoma. Additional nutrient sources were urban storm water runoff and runoff water from farm land, golf courses, and the Oklahoma City Zoo. Other potential sources of pollution observed during this study included wash water from animal pens at the zoo; leaking sewage lines along Eastern Avenue in Oklahoma City; runoff water from the construction of a horse racing track, dairy farm waste, field crops, and pecan orchards. There are 11 discharges into the river from industrial point sources, with four occurring in the headwaters of Lake Arcadia. There are 17 municipal wastewater discharges to the Deep Fork, nine in the headwaters above Lake Arcadia. At one time there were discharges from the Belle Isle power plant cooling-water lake. The environmental factors we observed were urbanization, dam construction, bridge construction, flooding, dam discharges, and agricultural runoff water.
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
Table 1 list the ranges of the water quality parameters in the Deep Fork River during this study. In the past, 21 water parameters exceeded recommended levels (5).
Approximately 40% of the upper basin lies within Oklahoma County and Oklahoma City. Site I, near Arcadia, experienced nutrient loading of 59,020 kg nitrogen per year (N/yr) and 181,600 kg phosphorus per year (PO4-P/yr). The waste water from the STP discharges contributed 526,640 kg N/yr and 149,820 kg PO4-P/yr. After dam construction and the diversion of STP effluent, there was an 80% reduction in nitrogen and phosphorus loading (6).
After the removal of the two STPs from the basin, there were major improvements in Kjeldahl nitrogen; total calculated nitrogen; total phosphorus; chlorides, potassium, sodium, organic carbon, and chemical oxygen demand (7). The water quality parameters that declined during construction were total iron, turbidity, suspended solids, total hardness, and calcium hardness. After construction, additional improvements occurred in most parameters because the lake acted as a nutrient trap (Table 1).
Toxicity surveys of the Deep Fork River indicated elevated levels of toxins in the water column, sediment, and fish tissue (7). In fish tissue collected before the impoundment of Lake Arcadia we found elevated levels of lead (133 µg/L) (8), mercury (1.7 µg/L) iron (81 mg/L), silver (8 µg/L) copper (27 µg/L), zinc (145 µg/L), DDT, and chlordane. After impoundment, we found elevated levels of chlordane (984 µg/kg), DDT (103 µg/kg), PCB (245 µg/kg), and mercury (0.37 µg/kg), in fish tissues (Unpubl. ODEQ data). Chlordane (FDA level 300 µg/kg), was the only toxin exceeding FDA human health warning levels.
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
After the impoundment of Lake Arcadia, we observed long periods of low flow, during which we recorded a reduction in depth and velocity, but mean water temperature was much higher (Table 1). Changes in stream bank stability, aggradation and degradation of the bed, and armoring of the substrata occurred soon after impoundment.
The flow regime of the Deep Fork River underwent three major changes. From 1970 to 1981, before the construction of Lake Arcadia, discharge was controlled by the Oklahoma City North Side STP, which released daily 41,635 m3, 28% of the daily discharge. During the construction period, 1982-1986, flows were more variable during the period after the removal of the STP from 0.09-4,767 L/s. The minimum flows were smaller. Flows were now controlled by rain events, and flow data indicated the most natural flow period, reflecting both droughts and flooding events. In the post-impoundment period, 1986-1996, flow was regulated by discharges from the lake. There were longer period of no measurable flows and long periods of elevated flows (Table 2).
{Page 73}
The post-construction period produced more flood-events, >472 L/s, 19.4 d/yr; during the pre-construction period, there were 4.5 d/yr. The largest discharges were recorded after impoundment and were produced by holding flood waters and releasing them over a long period (Table 2).
These long periods of elevated flows have caused many lake fish to escape from Lake Arcadia and subsequently to congregate at Site 1. Most of the Lepomis sp. and Pomoxis sp. captured at Site 1 were adults. Because high flows wash away gravel needed for nests and eggs, if these high flows occur during the fishes reproduction cycle, the scouring actions of long periods of high flow may account for this lack of young-of-the-year fish.
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
The first known fish collection in the drainage, 800 specimens, was in 1926 by Edith Force from the ponds and streams of Okmulgee County (Table 3). This survey lists 13 species. The specimens were identified by, and representative specimens deposited in, the United State National Museum in Washington, DC. Duplicate specimens were retained at the High School Museum in Okmulgee, OK; the University of Oklahoma Museum of Zoology, in Norman; and the University of Michigan Museum of Zoology, in Ann Arbor (9).
On 24 March 1932, A. Trowbridge and E. Strode collected nine species from Adams Creek, 0.8 km west of Preston, a tributary of the Deep Fork River in Okmulgee County and from the Deep Fork River 1.6 km from Okmulgee near an old pumping station. Later on 24 March 1935, they collected in Duck Creek, 0.8 km southeast of Mounds, below the spillway of Lake Okmulgee, 11.2 km southwest of Okmulgee, Okmulgee County (UMMZ museum records).
A. Houser and H. Lindsay of ODWC conducted the first extensive survey of the Deep Fork River during the pre-impoundment survey of Lake Eufaula (Table 4) (Unpub. UOMNH data). In 1964, they collected fish from six mainstem, seven tributary, and two lake sites and found a total of 46 species (UOMNH). In 1973, R. Miller sampled in Soldier, Coffee, and Coon Creeks near Arcadia and collected nine species; two were not found in the earlier survey (OSUS). Personnel from the Oklahoma City County Health Department (OCCHD) collected 23 species from three sites in the mainstem and two sites in Coffee Creek and added another species (Unpubl. OCCHD data). In 1991, during their survey for the Arkansas River shiner, R. Larson et al. made seven collections from the mainstem and found 15 species, one not found in earlier surveys (10). In 1992, R. Lemmons and G. Schnell, during their survey for flathead catfish, made seven collections from the mainstem and one in Salt Creek and collected 36 species, two species not found by earlier surveys (Table 4) (11).
From the examination of past fish collections we found records of 52 species (Table 4). During our long-term sampling we collected 36 species from the three long-term ODEQ monitoring sites. In 25 collections from Lake Arcadia, Lake Arcadia stilling basin, Northeast Lake (Zoo Lake) , and from the Deep Fork Arm of Lake Eufaula, we found 34 species. We add six species, Dorosoma petenense, Hiodon alosoides, Carassius auratus, Ictiobus cyprinellus, Ictalurus furcatus, and Menidia beryllina, bringing the know species list to 41 (Table 5). From our three long-term stations we added three new species, Ctenopharyngodon idella, Notropis volucellus, and Pimephales tenellus. Lake surveys by ODWC since the early 1960s reported three additional species, Ictiobus niger, Minytrema melanops, and Stizostedion vitreum, and seven hybrid types. The lack of voucher species may limit the usefulness of these species (ODWC unpubl. data).
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
Recent records of fishes we collected in the Deep Fork River east of Oklahoma City are presented in Table 6. The present survey lists 10 family groups and 35 species. A total of 58 species are known from the Deep Fork River. Earlier surveys collected 16 species not collected in our present study. In the present study we collected 10 species that were not found by earlier collectors.
{Page 74}
A total of 195,926 fish representing 35 species were collected in the current study. A total of 20,200 specimens of 28 species was collected at Site 1, below Lake Arcadia; 68,553 specimens representing 29 species were taken at Site 2; and 107,175 specimens representing 35 species were taken at Site 3 (Table 6). Cyprinella lutrensis was the predominant species, representing 74.6% of the fish, followed by the Gambusia affinis at 15.6%, Notropis stramineus at 4.2%, and Pimephales vigilax at 1.9% (Table 7).
The present study covers 21 yr from 1976 to 1997. Sixteen fish community parameters were examined. Long-term values were calculated for most of the fish community parameters (Table 8).
When using all the data from a site as a single sample, the index of numerical diversity (ND) ranged from 1.15 at Site 2 to 1.49 at Site 1. The ND was zero at Site 1 during the first four visits because we found no fish, and the ND was 0-2.59 at Site 2. The maximum ND for the three sites was 2.55 for Site 1, 2.59 for Site 2, and 2.05 for Site 3. A small temporal trend was observed in the ND at Sites 1 and 2 between the periods PWQ and RFP (Table 9). Site 1 showed a substantial spatial trend over Sites 2 and 3. The mean ND at Site 1 was larger than other downstream sites.
The biomass diversity (BD) was usually larger than the ND value. When we calculated the BD values using total biomass for each site, the BD values varied from 2.53 at Site 2 to 3.19 at Site 3. The single BD values calculated during our study varied from 0.25 to 2.88. A small spatial trend in the BD at Site 1 occurred downstream during PWQ, but during CNF the trend downstream was variable (Table 9). Temporal trends declined between PWQ and RFP at Site 2, and substantial increase in BD occurred between PWQ and RFP at Site 2.
In the sampling program, an area of 2,000 m2 was sampled. Considerable spatial variation in fish density occurred downstream. We observed a substantial increase in fish density from the modified section of river downstream of Site 1 to less modified downstream areas Sites 2 and 3 (Table 10). There was a substantial spatial trend downstream in number density (DN) of fish: 49.0, 74.0, and 99.0 fish per 100 m2. A substantial temporal trend was observed at Site 1 from PWQ to CNF to REF: 4.0-33.0-38.0 fish/100 m2. At Sites 2 and 3 we observed a substantial increase in DN as the water quality improved, but there was a major decrease in DN at Sites 2 and 3 during the RFP 96.0-74.0 and 147.0-87.0. An examination of the DN variability indicated low values of 0.00 fish/100 m2 at Site 1 in 1976-77 to 409 fish/100 m2 at Site 3 in 1983.
Mean biomass density (DBM), g/100 m2 exhibited a substantial spatial trend downstream during PWQ and CNF, 6.0, 26.0, 58.0 g/100 m2. and 30.0, 45.0, 147.0 g/100 m2. During the CNF, Site 1 decline to Site 2, a substantial increase at Site 3 followed. The DBM varied from 0.0 g/100 m2 to 752.0 g/m2 (Table 10). The largest biomass of fish was collected on 1 July 1991 when we collected 14 large Carpiodes carpio, two large Ictiobus bubalus and a large Pylodictis olivaris. The DBM was 752.0 g/100 m2 at Site 3.
The mean biomass (MBM) exhibited a substantial temporal trend at Sites 1 and 2. Both sites had an increase in the MBM, PWQ<CNF<RFP (Table 11). At Site 3 a visible increase occurred PWQ<CNF, but leveled off when CNF=RFP. The largest MBM was 6.7 kg of fish at Site 3 in 1991. A substantial spatial trend was visible at all three stations during the PWQ and CNF, Site 1<Site 2<Site 3. During the REF period, it appears Site 1>Site 2<Site 3 exhibited no trend downstream.
We observed a substantial increase in the average abundance, Site 1<Site 2<Site 3. The values were 71, 155, and 206 specimens per species, respectively. The range varied widely, from three specimens per species per collection to 1,276 specimens per species per collection (Table 8).
The mean weight W for individual fish was much greater at Site 1, 2.7 g/fish. At Sites 2 and 3 W declined from 0.8 g/fish and 1.1 g/fish, respectively. The mean weight per fish showed a substantial decrease downstream from Site 1. During the PWQ period, Site 1>Site 2>Site 1 exhibited a decline of 2.4, 1.4,
{Page 75}
and 1.3 g/fish, respectively. During CNF we observed Site 1>Site 2=Site 3. In the RFP, Site 1>Site 2<Site 3. The range in weight for specimens varied from 0.1 g/fish to 214.9 g/fish. On 8 May 1989, we collected 17 specimens with a weight of 4,942.7 g from Site 1 (Table 11).
Using our fish community rating index (FCRI), we calculated the condition of the fish community at each site of the river (12). Using data for the entire period of the study, the rating values varied from 114 to 129, and the spatial trend indicated a fair fish community. The widest range in rating occurred at Site 1 where the values varied from very poor, no fish at 299, to fair at 123. The average value of the FCRI for all three sites was very similar Site 1 fair, at 153; Site 2 fair, at 162; and Site 3, fair at 156 (Table 12).
A significant temporal trend in FCRI was observed when (PWQ)>(CNF)>(REF), P £ 0.01 (Table 13). The major temporal trend, 208-173-139, occurred at Site 1 during our survey (Table 12). At Sites 2 and 3, the trend exhibited was (Site 1=Site 2)>(Site 2=Site 3), P = 0.05. We observed only a small change, 161-167-156 and 160-160-149 in the FCRI (Fig. 2). The interactions between sites and time was very significant, P = 0.001.
Poor water quality, lack of suitable aquatic habitats, and changes in flow patterns have helped to limit the number of families found in the Deep Fork River (Table 14). Two families are rare in the Deep Fork River. Percidae were not found during our survey, and we found only two species of the Catostomidae. The Cyprinidae were represented by 12 species, and the Centrarchidae by 10.
We collected three intolerant species during our survey: Phenacobius mirabilis, Pimephales tenellus, and Notropis volucellus. Phenacobius mirabilis were collected in small numbers, 150 specimens, while N. volucellus and P. tenellus were very rare. We based our listing of intolerant species on the work of D. Jester et al. (13)
We collected small numbers of four introduced species: grass carp, common carp, inland silversides, and redear sunfish. Carp were the most abundant introduced species. The grass carp was the only new introduced species for the river. The Oklahoma Department of Wildlife Conservation (ODWC) stocking records indicate the introduction of inland silversides, walleye, and threadfin shad into lakes throughout the drainage. Two hybrids were also introduced by ODWC, the saugeye and hybrid striped bass.
Sport fish were common throughout the drainage. We collected 12 sport species at Sites 1 and 2; at Site 3 we collected 12 species and five hybrids. Because of the high steep banks, there was limited access for fishing at all three sites. At Site 2, there was some sport fishing for channel catfish and flathead catfish. Several sport species occurred in the drainage, but did not occur in our sampling sites: Morone chrysops, Stizostedion vitreum, hybrid striped bass, and saugeye.
Rough fish species occurred at all three sites. A large number, 791 specimens, was collected at Site 3. One collection had 253 rough fish. The most abundant rough fish species was the gizzard shad, which was found at all three sites. Small numbers of carp, river carpsuckers, and smallmouth buffalo were collected. We also collected one shortnose gar at Site 3 (Table 7).
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
The abundance and distribution of fish at each station are presented in (Table 6). The red shiner, mosquito fish, sand shiner, and Pimephales vigilax, bullhead minnow, were collected at every station. The large numbers of young and juvenile Cyprinella lutrensts, or red shiners, and Notropis stramineus, sand shiners, were interpreted as an indication of these shiners, successful reproduction throughout the study area. Both shiners were taken in most of the collections and were present in 108, 105, and 112 of the collections from the three sites, respectively (Table 7).
Cyprinids fishes were the most abundant taxa collected, comprising 86%, 90%, and 78% of the fish collected at Sites 1 to 3 respectively. Three minnow species ranked in the five most abundant species collected: the red shiner (#1), sand shiner (#3), and bullhead minnow (#5, Table 7).
{Page 76}
Insectivorous cyprinids fish comprised 85% of the fish collected at Site 2. At Sites 1 and 3, this group comprised 74% and 73%, respectively. Omnivorous cyprinid fishes comprised 4-12% of the fish collected at Sites 1 and 3, respectively.
The examination of the food tropic levels revealed that the insectivores were more numerous than the omnivorous. The largest percentage of top-order carnivorous fishes were found at Site 1. This reflects the escape of carnivorous fishes from the lake and their assemblage below the lake. Herbivorous fish comprised a small percentage of the fish collected, represented by a single species, the Dorosoma cepedianum (Fig. 3).
Indices of similarity were computed to determine the similarity in species occurrence between sites and yearly collections. The S indices of the Deep Fork fish community for the three mainstream sites indicate that the stations were very similar. High similarity, >0.80, was observed between some stations. The highest similarity, 0.90, was between Sites 2 and 3. The lowest observed similarity 0.79, was between Sites 1 and 3. The high similarity between Sites 2 and 3 was as we anticipated. Adjacent stations would be expected to have the highest similarity due to the vagility of most fish species (Table 15).
Collecting with seines, electrofishing, and gill nets, we collected one specimen of one species of gar. We found gar were very rare in the upper Deep Fork River. In 1988, a Lepisosteus platostomus was collected at Site 3. Other species, which were too rare to establish distribution trends, were Ctenopharyngodon idella, Notropis volucellus, Pimephales tenellus, Lepomis gulosus, and Lepomis gulosus (Table 7).
Darosoma cepedianum were present for 14 yr, but were not collected at Site 1 until 1985. This species has shown an irregular pattern of occurrences at Site 1 since then. The number of shad collected at Site 1 was variable, 1-27 specimens. The suckermouth minnows are more abundant today in our collections than in the past. This species was not found at Site 1 until 1988 and was more abundant at the downstream sites, Sites 2 and 3. Inland silversides were captured in 1980-81 from Site 3, and again in 1992-93 from all three sites. These were escapees from Lake Arcadia where they were stocked by the ODWC (Unpubl. ODWC records).
These species were collected every year since 1978: Cyprinella lutrensis, Notropis stramineus, Fundulus zebrinus, Western mosquitofish, Lepomis cyanellus, Lepomis macrochirus, Lepomis megalotis, Micropterus salmoides. However, the plains killifish has not appeared at Site 1 since 1990. It has occurred in small number at Sites 2 and 3, where it is still being collected today.
Since 1978, Cyprinus carpio were collected every year except 1986. But they were not found at Site 1 until 1983. Common Carp are very common in downstream sites, and many probably escaped from Lake Arcadia, which supports a large population of carp. The Pimephales promelas was collected every year, but not from all sites each year. This species has been on the decline since 1990, which may be due to the increase in numbers of top carnivores at Site 1. We observed that this minnow had disappeared from Site 1 in 1990.
The Lepomis humilis was collected every year except 1982. This species was first collected at Site 1 in 1985. The Lepomis microlophus was uncommon and was found in six annual collections. The largemouth bass was found in 13 annual collections, but was rare until 1985. Since 1976, this species is more abundant at all sites. This increase may represent escapees from the lake. Since 1985, the Pomoxis annularis has occurred every year and was found at most sites. This is another species that represents escape from the lake. The Pomoxis nigromaculatus was found in 10 annual samples and occurred in small numbers at most sites. Aplodinotus grunniens were found in six annual collections since 1985 and were found only at Sites 2 and 3. We have not collected this species from Site 1.
The Hybognathus placitus has never been collected at Site 1, but has occurred at Site 3 since 1984. This species has been on the decline since 1991 and has been collected twice since 1984. Notemigonus crysoleucas were un-
{Page 77}
common. Since 1995, they have been found only at Sites 2 and 3. The Notropis atherinoides is on the decline and has not been collected since 1986, the year that regulated flows began.
A species on the increase was the bullhead minnow, which has been collected for 13 y since 1980, but collected just twice at Site 1 since 1976. Carpiodes carpio and Ictiobus bubalus were more abundant downstream where we found them in deep, instream pools. These species were limited to Sites 2 and 3. We failed to collect these two species at Site 1 during the survey. The smallmouth buffalo has not been collected since 1990.
Ameiurus melas were rare in the river until 1984. An increase in numbers was observed between 1984 and 1989, which may reflect the large numbers of escapees of this species from Lake Arcadia. Ameiurus natalis were rare. They were only found in four yearly collections, first in 1981. The Ictalurus punctatus has been collected every year since 1982. Pylodictis olivaris were uncommon in the river and were found seven times, usually at Sites 2 and 3, but not from Site 1.
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
The number of families and species in collections reflected major environmental changes. At Site 1, the most impacted site, there were substantial increases in both the number of families and species during the period of study. The numbers by periods indicate substantial improvement after the PWQ period, from 1.4 families before 1981, to 3.4 families between 1981 and 1985, to 4.1 families between 1985 and 1996, the post-impoundment period. At Sites 2 and 3, the number of families were greatest during the CNF (Table 14).
A small spatial increase was observed at all three Sites during each of the three major periods. The total number of families increased from 6.0 at Site 1, 8.0 at Site 2, and 10. 0 at Site 3, respectively. The greatest difference in total number of families collected was between Site 1, with 6.0, and Site 3, with 10.0. A substantial difference was observed between Sites 1 and 2 and 3 in families per collection during the PWQ period (Table 14).
A substantial temporal improvement in the number of species per collection was observed for Site 1. For the three time periods the values were 3.8 species for PWQ, 6.9 species for CNF, and 10.9 species for RFP (Table 14). Sites 2 and 3 exhibited similar improvements during CNF with values of 10.0 and 11.4 species/collection respectively. However, during the RFP of flushing out flows there was very little change in the number of species at Site 2, from 9.0 to 8.9. At Site 3, the number of species per collection increased from 11.4 to 12.1.
During the PWQ, 1976-1980, there was a substantial spatial trend between Sites 1 and 2 and Sites 2 and 3 in the number of species collected. During the CNF, we observed a major improvement in the number of species per collection at Sites 1 and 3. There was a small increase in the number of species per collection at Sites 2 and 3 during the RFP. Examination of the species by period showed a significant temporal trend (PWQ£CNF£RFP), P = 0.01 (Table 16).
As expected, we observed a small spatial trend in the total number of species collected at each site (Fig. 4). At Sites 1 and 2 we found 26 and 27 species, respectively. At Site 3 we collected 34 species, when (Site 1<(Site 2=Site 3), P £ 0.05, would suggest the significance of this trend between sites (Table 16). The significance of P £ 0.01 and P = 0.01 would show a temporal trend between PWQ-RFP and PWQ-CNF (Table 17).
A substantial temporal improvement was observed in the FCRI at Site 1 from a value of 208, which was a very poor rating during PWQ, to 173, fair for the CNF, and to 139, fair during RFP. Sites 2 and 3 showed improvements throughout the period of the study (Table 13). However, this degree of improvement was smaller than that for Site 1 (Fig. 2). At Site 2, the FCRI was 161-167-155, respectively, for PWQ, CNF, and RFP periods of major environmental changes. All FCRI ratings were fair. At Site 3, the FCRI ratings were 160 for PWQ 160 for CNF, and 147 for RFP, a fair rating also (Table 11).
{Page 78}
A similar pattern of spatial improvement was observed between Sites 1 and 3. During the period of PWQ, Site 1, with its rating of 200, was a very poor fish community and was much lower than Site 2, with an FCRI rating of 161 (fair) and Site 3, with an FCRI rating of 160 (fair). However, during the CNF we observed that the FCRI was very similar for all sites. At Site 1 the FRCI was 173 (fair). At Site 2, the FRCI was 167 (fair) and at Site 3 it was 155 (fair). This improvement in FCRI is related to the return to a more natural flow and improved water quality during the construction period. A combination of escaped fish from the lake and the concentration of fish below the lake helped to contribute to a substantial change in the FCRI of 139 (fair) at Site 1 during the RFP (Table 12). A smaller improvement in the FCRI was observed at Sites 2 and 3. The values were 155 (fair) at Site 2 and 147 (fair) at Site 3. The temporal trends between the periods (PWQ)>(CNF)>(RFP), P £ 0.01, and in the spatial trends between sites (Site 1)=(Site 2)>(Site 2=Site 3), P = 0.05 were significant (Table 13). The interaction between time and space showed a significance of P = 0.001. This indicates a significant improvement in FCRI.
Between 1976 and 1980, we observed at Site 1 an average of 35 specimens per collection. Between 1980 and 1985, we found an average of 499 specimens per collection. During the period of RFP, the number improved to 1,506. We observed similar changes in the number of specimens at Sites 2 and 3 (Table 18). The largest average number 5,754 specimens per collection was collected in 1983 from Site 3.
We also observed during the same time the numbers of specimens per collection varied downstream from 35 specimens at Site 1, to 738 specimens at Site 2, and 1,018 specimens at Site 3 (Fig. 5). Similar observations were made during CNF: 499 specimens at Site 1, 1,613 specimens at Site 2, and 2,934 specimens at Site 3. Large numbers were found during the RFP: 1,506, 2,862, and 5,123 specimens from Sites 1, 2, and 3, respectively. A significant temporal trend was observed (PWQ)<(CNF=REF), P £ 0.05, and a significant spatial trend was observed when (Site 1)<(Site 2=Site 3), P £ 0.05 (Table 17).
The percentage of red shiners varied widely (Fig. 6), but we could not observe a well defined temporal or spatial trend. During all three periods, Site 2 had the highest percent 74.7%-85.8%-76.3%, of red shiners, and all sites show the largest percent of red shiner during the CNF. The PWQ period had a smaller impact on the percentage of red shiners.
On four occasions, the yearly average percent of red shiners comprised over 90.0% of the fish collected. Three of these high percentages occurred at Site 2: 91.9%, 96.6%, and 98.1% in 1979, 1983, and 1984, respectively. Once at Site 3, in 1988, red shiners comprised 90.4% of the collected fish. On four occasions red shiners comprised less then 50.0% of the fish. Three of these occasions occurred at Site 1 : 48.4% in 1978, 36.8% in 1989, and 44.2% in 1994. One low percent, 46.6% was observed at Site 3 in 1982.
We observed a significant temporal trend in the number of red shiners collected during the three periods, (PWQ)<(CNF=RFP), P £ 0.01. A significant, (Site 1)<(Site 2=Site 3) P £ 0.03, spatial trend occurred in the numbers of red shiners downstream (Table 20)
During the period of PWQ, there were low mean similarities, P £ 0.50, between fish communities at Site 1 and Sites 2 and 3 (Table 15). The mean similarities between sites were almost the same 0.47 and 0.48.
During PWQ period however, mean similarities were moderate 0.63, between Sites 2 and 3. During the next two periods, CNF and RFP, the mean similarities between Sites 1 and 2, 0.63 and 0.62, and 1 and 3, 0.62 and 0.66, were moderate. The similarities between Sites 2 and 3 increased slightly from period to period 0.63 to 0.70 to 0.73. Throughout the period of the study, Sites 2 and 3 showed a small temporal improvement in similarities between the sites. The highest similarity observed was 0.90 in 1985 between Sites 1 and 2 near the end of the CNF. The smallest similarity was between Sites 1 and 3 in 1980 during the period of PWQ.
Similarity between collections for Site 1
{Page 79}
varied widely from 0.92 to 0.00 (Table 15). At Site 1, a comparison of collections made during each period of environmental stress was usually more similar than those comparisons between collections made later. The low similarities during PWQ, CNF, and RFP between collection indicated a major improvement with time. Collection similarities between sites exhibited low similarities between PWQ and PWQ, 0.56 to 0.60; PWQ and CNF, 0.51 to 0.55; and PWQ and RFP, 0.43 to 0.36. However, similarities between collections within each of the periods showed moderate to high similarities: PWQ and PWQ 0.56 to 0.60; CNF and CNF, 0.50 to 0.64, and RFP and RFP, 0.70 to 0.87. These high similarities would indicate that a major improvement in species richness had occurred at Site 1 (Table 15).
The similarities between collections at Site 2 were fairly high. Between CNF and RFP the similarity was moderate, 0.59 to 0.69. Within each period the similarity was fairly high between CNF and CNF, 0.63 to 0.74, and RFP and RFP, 0.65 to 0.87. These values indicate a fairly stable species richness between collections during the study (Table 15).
Site 3 similarities between collections were usually high. Comparison between collections during each period showed the S between PWQ and CNF was 0.73 to 0.75, PWQ and RFP was 0.67 to 0.67 and CNF and RFP was 0.59 to 0.77. Similarities between collections during each period were moderately high to high: PWQ and PWQ, 0.69 to 0.86: CNF and CNF, 0.71 to 0.86: and RFP, and RFP 0.63 to 0.78. The decline in S at Site 3 may reflect a decline in species richness between collections due to changing flow patterns or seasonal movements of some species.
Declines were observed in the levels of toxic substances in the tissue of the fish in the river during RFP. During the PWQ, substantial levels of copper, chromium, chlordane, and PCBs were found in fish tissue from Site 3. During the CNF period the mean levels of mercury, chromium, chlordane, and PCBs declined. However, copper increased from 0.200 mg/kg to 1.162 mg/kg. The greatest decline occurred during the CFP. Lake Arcadia was serving as a trap for toxic substances. Fish tissues from Lake Arcadia had elevated levels of mercury and chlordane. During RFP, all toxic substances in the fish tissue from the river declined. The chlordane and PCBs declined to below the detectable limits for these toxic substances. Mercury, however was still occurring above detectable limits in some fish tissues (Unpublished DEQ data).
The substrata at Site 1, before regulation in 1988, consisted of a very fine, reddish, sandy silt. Now there is a large area of waste concrete and rock, which comes from an old bridge, that forms a very stable substrata. The silt was very deep, 0.3-0.4 m, in the pools and 0.1-0.2 m deep in the riffles. After the construction of Lake Arcadia, the altered flow regime changed the substrata at this site. The flushing out actions on the riffles cut down to the red hard-pan clay, red clay, and in some cases, to the shales. The pools were first filled in and later flushed out, forming small shallow pools that resulted in a uniform habitat lacking instream cover and gravels for reproduction. At Site 3, we observed that a similar change in habitat due to the extended periods of high flows and continuous discharge from the lake has also caused major habitat changes that reduced pool habitats. The loss of pool habitat has reduced several species that require deep pool habitat.
The number of Centrarchids (Table 21) for each collection exhibit a significant temporal trend (PWQ=CNF)<(RFP), P £ 0.05. They also exhibited a significant spatial trend (Site 1=Site 2=Site 3), P £ 0.05 (Table 22). The interaction between spatial and temporal factors exhibited a significant relationship, P = 0.0001 (Figure 7).
The number of Lepomis megalotis per collections (Table 23) exhibited significant (PWQ=CNF)<(CNF=RFP), P £ 0.05 temporal trends. The interaction between site and time yielded a significant relationship, P = 0.001. The longear exhibited a major increase in numbers during RFP (Fig. 8).
The number of Gambusia affinis exhibited a significant change over time (PWQ=RFP)<(CNF), P = 0.01 (Table 17), and a significant spatial trend (Site 1=Site
{Page 80}
2)<(Site 3), P = 0.05. This species exhibited a significant increase during CNF (Table 24) and a downstream decrease between sites during CNF and RFP during periods of regulated flows (Fig. 9).
The number of Notropis stramineus per collection did not exhibit a significant trend in either time or space. This species increased in numbers downstream during the CNP (Fig. 10). But declined in numbers downstream during RFP (Table 25).
The water quality in the Deep Fork River downstream from storm runoff waters, domestic discharges, and dam releases has greatly improved in the 20 y since this study started. In 1976 and 1977, no fish were collected at Station 1 downstream from those effluents. Station 2 was severely stressed, and only partial recovery was observed. After the construction of Lake Arcadia and the upgrading of the water quality in 1988, similar numbers of these fishes and species found at Sites 2 and 3 were also found at Site 1. However, fewer fish were taken per collection. The Deep Fork River contains native fish communities dominated by prairie fishes that are relatively pollution tolerant. The changes in the native fish communities from major environmental changes were softened because of the tolerant species of fish. Changes from these stresses were usually much smaller then expected.
Field work for this study was made possible by the Oklahoma State Department of Health and Oklahoma Department of Environmental Quality. Administrative support was by Mr. Mark Coleman, Ms. Judith Duncan, and Steve Houghton. Mr. Tim Beard, Mr. Waymon Harrison, Mr. Mike Petzell, Mr. David Holcomb, Mr. Thai Pham, Mr. Randy Parham, Mr. Phil Pigg, Mr. Geron Cottom, Mr. Rich Sawin, and Mr. Jamie Jones assisted in the collection of fish samples. A special thanks goes to Mr. Aaron Mitchum and unknown reviewers for their help in reviewing and improving this manuscript, to Ken Cunningham for his help with analysis of data and the outstanding job of editing by Miss Carrie McDowell.
| Introduction | Description of Deep Fork River | Methods | Site Selection | Description of Sampling Stations |
| Environmental Influences | Water Quality | Flow Regime | Historical Fish Collections | Results & Discussion |
| Species Abundance and Distribution | Influences of Environment stresses on Fish Community Parameters | References | Table of Contents | Home |
1. Brinkley FJ. Biological studies, Ohio River pollution survey. I. Biological zones in a polluted stream. Sewage Works J 1942;14:147-159.
2. Blazs RL, Walters DM, Coffey TE, White DK, Boyle DL, Kerestes JF. Water resources data water year 1994. Volume 1. Arkansas River Basin. USGS Water-Data Report Ok-94-1. (1994).
3. Wilhm J. Species diversity of fish populations in Oklahoma Reservoirs. Ann Okla Acad Sci 1976;5:29-46.
4. Odum EP. Fundamentals of Ecology. 3rd ed. Philadelphia (PA): WB Saunders Co; 1971. 144 p.
5. U.S. Army Corps of Engineers, District, Tulsa, Oklahoma. Arcadia Lake water quality evaluation. Environmental Effects Laboratory. U.S. Army Engineer Waterways Experiment Station. Vicksburg, Mississippi; 1974. 49 p.
6. Department of the Army, Tulsa District, Corps of Engineering, Tulsa Oklahoma. Draft. Updated and revised environment statement, Arcadia, Deep Fork River, Oklahoma; 1974. 132 p.
7. Wright JC. The detection of trends and changes in streams and rivers in Oklahoma. [MS thesis]. Norman (OK): University of Oklahoma; 1994. 210 p. Available from University of Oklahoma Library.
8. Pigg J, Coleman MS, Roach B. Distribution of lead in the upper Deep Fork River System in Oklahoma. Proc Okla Acad Sci 1975;55:19-24.
9. Force ER. Fish of the ponds and streams of Okmulgee County. Proc Okla Acad Sci 1927;7:137.
10. Larson RD, Echelle AA, Zale AV. Life history and distribution of the Arkansas River shiner in Oklahoma. Project final report. Oklahoma City (OK): Department of Wildlife Conservation; 1991. Oklahoma.
{Page 81}
11. Lemmons RP, Schnell G. Flathead catfish ecology and population structure in Oklahoma Prairie Streams. Project final report. Oklahoma City: Oklahoma Department of Wildlife Conservation; 1994. Oklahoma
12. Pigg J. Aquatic habitats and fish distribution in a large Oklahoma river, the Cimarron River from 1976 to 1986. Proc Okla Acad Sci 1988;68:9-31.
13. Jester DB, Echelle AA, Matthews WJ, Pigg J, Scott CM, Collins K D. The fishes of Oklahoma, their gross habitats, and their tolerance of degradation in water quality and habitat. Proc Okla Acad Sci 1992;72:7-20.
Received: 1997 Nov 20; Accepted: 1998 Jul 10.