
|
 |
|
{Page 141}
Department of Chemistry and Biochemistry, University of Oklahoma, Norman, OK 73019
In some addition reactions of acyclic alkenes, plots of ionization potential (IP) versus the log of relative rate show a natural grouping of data points corresponding to the number of alkyl groups attached to the alkene carbons. Plots corresponding to the reaction of Cl2, Br2, and I2 with representative alkenes are presented; similarities and differences among the reactions are discussed. A plot of the I2 data exhibits a natural separation into groups of similarly-substituted alkenes, in which increased substitution reduces the rate. Within the groups of similarly-substituted alkenes, a good-to-excellent correlation is observed, with a lower IP generally corresponding to a higher relative rate. The Cl2 data and Br2 data each show a similar correlation but without separation into similarly-substituted groups. Increasing substitution increases the reaction rate where Cl2 or Br2 is employed, in contrast to cases in which I2 is employed. ©2000 Oklahoma Academy of Science
| Introduction | Results and Discussion | References | Top of Page | Table of Contents | Home |
We have developed a simple procedure for ascertaining whether additions to acyclic alkenes exhibit steric effects which are either dependent on the degree of substitution about the double bond, or are of the same order of magnitude regardless of the degree of substitution about the double bond (1-5). Such information improves the understanding of the reaction and its synthetic usefulness. In this procedure, (a) relative rates of reaction of a number of representative alkenes, with a broad range of electronic and steric properties are determined; (b) log krel (log of the relative rate compared to that of the reference alkene) of each alkene is plotted against the alkene þ ionization potential (IP); and (c) plots and correlation coefficients are examined for linearity and number of lines, with each line representing a group of alkenes having steric effects with similar orders of magnitude in that reaction. Alkene IPs are used for comparison because they are relatively insensitive to steric effects.
We previously contrasted bromination of alkenes against hydroboration and oxymercuration (1) and compared it to a number of reactions of alkenes involving 3-membered intermediates (2). We determined that hydroboration and oxymer-curation each have multiple lines in the plots, indicating they each have groups of alkenes with steric effects which are of the same order of magnitude within groups, but different from one group to another (1). However, bromination gives one line for all alkenes, indicating that steric effects in that reaction are of the same order of magnitude for those alkenes studied, similarly to most of the other reactions involving three-membered intermediates (2). In order to explore the effectiveness of the procedure, it seemed desirable to compare bromination to the reactions of alkenes with chlorine and with iodine; therefore, we report the results of that comparison.
For chlorination, in nonpolar media and free from radical contributions (7), relative rates parallel those of bromine addition.
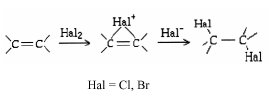
{Page 142}
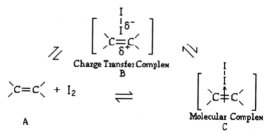
Studies of alkene iodination explored
adsorption (A B) of the olefin with solid iodine on a GC column to give a
charge-transfer complex B and complexation
(B
C) from the adsorbed alkene B to give the molecular complex C
(12).
| Introduction | Results and Discussion | References | Top of Page | Table of Contents | Home |
Table 1 lists relative rates of reaction (krel) of representative acyclic alkenes with chlorine (7), with bromine (8-11), and with iodine (12) and also lists alkene IPs and highest occupied molecular orbital (HOMO) energy levels. Relative reactivities in the table are relative to each other, with 1-hexene selected as the reference and given the value of 100. In some cases, IPs were not available and had to be determined through comparison with HOMOs, which were calculated as described previously (2). Plots of alkene IPs versus log krel values are shown in Figures. 1-3. Since a higher IP corresponds to electron removal from a lower-energy molecular orbital, IPs were plotted in inverse order on the y-axis of each plot, in order to make the plots comparable to those using HOMO energy levels. These plots reveal results for chlorination (one line of correlation with the correlation coefficient, r = 0.99) which are very similar to those for bromination (one line of correlation with r = 0.97).
Results for iodination (12) are more complicated. Studies were carried out in order to observe the interaction of olefins with molecular iodine by using a gas chromatographic (GC) technique. The results were analyzed in order to explore both absorption and complexation of the olefin with iodine on the column. Each set of data was further treated mathematically in two ways: (a) accounting for complexation with untreated support and (b) not accounting for complexation with untreated support.
The plots for reaction with iodine are unlike those for chlorination and for bromination. In treatments that allowed complexation with the support, adsorption and complexation with iodine gave conflicting results. Adsorption showed a grouping according to the amount of steric hindrance, whereas complexation shows no such grouping. However, the level of correlation in these plots is only moderate.
When complexation with support was not allowed, again adsorption showed the grouping and complexation does not (Figures 3a-d). The level of correlation in these plots is much higher, especially for adsorption, which has a correlation coefficient of 0.8 and 0.7 for mono- and di-substituted alkenes, respectively. The manifestation of grouping for adsorption, but not for complexation, is surprising, because adsorption should be a much "looser" interaction with the molecules farther apart.
At first the different results might seem surprising, but they are easily understood. Bromination and chlorination are addition reactions which go to completion. The reaction with iodine does not go to completion because it is unfavorable entropically and is endothermic; it is a reversible complexation reaction. Although the iodonium ion is presumably formed, the equilibrium favors the reactants. Therefore, the plot obtained from reaction with iodine might be expected to resemble that of alkene complexation with silver ion (AgNO3) (2). Comparison of the two plots does indeed reveal an obvious similarity. Each plot has multiple lines, with positive slopes and good-to-excellent correlations, and groups the alkenes according to their steric requirements.
| Introduction | Results and Discussion | References | Top of Page | Table of Contents | Home |
1. Nelson DJ, Soundararajan R, Cooper, P. Simplified method of ascertaining steric effects in electrophilic addition reactions. A comparison of bromination , oxymer-curation, and hydroboration. J. Am. Chem. Soc. 1989; 111: 1414.
2. Nelson DJ, Soundararajan R. Using the comparison of steric versus electronic
{Page 143}
Page 143 consists entirely of Table 1, Figure 1 and Figure 2
{Page 144}
Page 144 consists entirely of Figure 3
{Page 145}
effects to infer mechanistic information in stepwise electrophilic addition reactions involving three-membered cyclic intermediates. Tetrahedron Lett. 1988; 29: 6207.
3. Nelson DJ, Yao Z, Henley R, Smith T. Diimide reduction of representative alkenes and correlation of their relative reaction rates with corresponding ionization potentials. Tetrahedron Lett. 1993; 34: 5835.
4. Nelson DJ, Henley R. Relative rates of permanganate oxidation of func-tionalized alkenes and the correlation with the ionization potentials of those alkenes. Tetrahedron Lett. 1995; 36: 6375.
5. Nelson DJ, Perng T, Campbell D. Relative magnitudes of steric effects in reactions of Cl2, of Br2, and of I2 with representative acyclic alkenes. Proceedings of the 26th National Triennial Convention of Iota Sigma Pi. Iota Sigma Pi Promethium Chapter: Portland, OR, 1999.
6. Masclet P, Grosjean D, Mouvier G, Dubois J. Alkene ionization potentials, Part I: Quantitative determination of alkyl group structural effects. J. Electron Spectrosc. Relat. Phenomena 1973; 2: 225-37.
7. Poutsma ML. Chlorination studies of unsaturated materials in nonpolar media. IV. The ionic pathway for alkylated ethylenes. Products and relative reactivities. J. Am. Chem. Soc. 1965; 87: 4285.
8. Collin VG, Jahnke U, Justm G, Lorenz G, Pritzkow W, Rolling M, Winguth L, Dietrich P, Doring CE, Houthal HG, Wiedenhoft A. Kinetik einiger elektrophiler olefin-additionen. J. Prakt. Chem. 1969; 311: 238.
9. Dubois JE, Walisch W. Kinetische untersuchungen über die bromaddition an olefine. Chem. Ber. 1959; 92: 1028.
10. Dubois JE, Barbier G. Etude cinetique de l'addition du brome sur les olefines- VII. Insensibilite des effets de structure. J. Prakt. Chem. 1965; 303: 1217.
11. Dubois JE, Mouvier G. Kinetic investigation of the addition of bromine to olefins. Influence of structure on the bromination velocity of olefins. C. R. Hebd Seances Acad. Sci (Paris) 1964; 259: 2101.
12. Cvetanovic RJ, Duncan FJ, Falconer WE, Sunders WA. Electron donor-acceptor interactions of molecular iodine with olefins. J. Am. Chem. Soc. 1966; 88: 1602.
13. Brown RS, Marcinko RW. Influence of substituents upon ionization potential. Dependence of the p-ionization energy on the orientation of an allylic hydroxyl or methoxyl substituent. J. Am. Chem. Soc. 1978; 100: 5721.
14. Nelson DJ, Dewar MJS, Buschek JM, McCarthy EJ. Effect of a mercuric sulfate precolumn on chloro olefin abstraction. J. Org. Chem. 1979; 44: 4109-4113.
15. Klasnic L, Ruscic B, Sabljic A, Trinajstic N. Application of photoelectron spectroscopy to biologically active molecules and their constituent. Parts. 6. Opiate narcotics J. Am. Chem. Soc. 1979; 101: 7477.
16. Willet GD, Baer T. Thermochemistry and dissociation dynamics of state-selected C4H4X ions. 3. C4H5N+. J. Am. Chem. Soc.1980; 102: 6774.
17. Schmidt H.; Schweig A. Semi-quantitative proof of hypercojugation. Angew. Chem., Int. Ed. Engl. 1973; 12: 307.
Received: March 20, 2000; Accepted: August 14, 2000.
| Introduction | Results and Discussion | References | Top of Page | Table of Contents | Home |