
|
 |
|
{Page 11}
Oklahoma Cooperative Fish and Wildlife Research Unit, Department of Zoology,
Oklahoma State University, Stillwater, OK 74078
Department of Botany, Oklahoma State University, Stillwater, OK 74078
Department of Biological Sciences, University of New Orleans, New Orleans, LA 70148
Department of Agronomy, Oklahoma State University, Stillwater, OK 74078
1Current address: Missouri Department of Conservation, Open River Field Station, 3815 East Jackson Blvd., Jackson, MO 63755
2Current address: Department of Zoology, Southern Illinois University, Carbondale, IL 62901
We studied vascular plant communities on eight black-tailed prairie dog (Cynomys ludovicianus) colonies in Cimarron County, Oklahoma. Our objectives were to determine whether plant species composition and richness varied within and among prairie dog colonies and to determine whether plant species composition varied between prairie dog colonies and surrounding grasslands. We hypothesized that prairie dog colonies in southern shortgrass prairie would support greater richness than surrounding shortgrass prairie and that differences would be detected between the edge and interior of the colony as a result of edge effects. Data were collected in May 1996, during drought-induced dormancy. We found a total of 42 species representing 13 families on the eight prairie dog colonies and adjacent prairie. Detrended Correspondence Analysis revealed species composition variation was primarily attributable to variation among colonies, rather than variation within colonies. Hence, there were no distinctions between prairie, edge, and interior habitat types. A Monte Carlo test, following a partial Canonical Correspondence Analysis, revealed there were no significant effects of location within a site on vegetation composition (P > 0.05). Our findings suggest that the black-tailed prairie dog may not function as a keystone species in southern shortgrass prairie. © 2001 Oklahoma Academy of Science
| Introduction | Materials and Methods | Results | Discussion | References | Top of Page | Table of Contents | Home |
The black-tailed prairie dog (Cynomys ludovicianus), a large burrowing rodent found in grasslands of the western Great Plains, once occurred over approximately 40 million ha before European settlement (1-3). Prairie dogs (C. ludovicianus, C. leucurus, C. gunnisoni, and C. parvidens) covered approximately 283 million ha in the late 1800s (4), but have been reduced to 2% of their former range, primarily because of eradication programs, human development of the Great Plains (5), and sylvatic plague (6).
Prairie dogs are recognized by some ecologists as keystone species in the Great Plains grasslands (7,8). Recent scientific discussions have helped fuel petitions by the
{Page 12}
Biodiversity Legal Foundation and J.C. Sharps as well as the National Wildlife Foundation to list the black-tailed prairie dog under the Endangered Species Act. Even though the U.S. Fish and Wildlife Service declined to list the species, the effort highlighted the urgency of understanding the role of prairie dog colonies in ecosystem function.
Most studies of vegetation on prairie dog colonies have been conducted within the northern mixed-grass region of the Great Plains (9), and most have focused on food habits of prairie dogs and dietary overlap with livestock (1,10). Studies on prairie dogs and their effects on vegetation in the southern shortgrass prairie are lacking (9). Hence, we agree with Winter and co-workers (11) that data are needed to help determine whether prairie dogs actually function as keystone species throughout all regions of the Great Plains.
We hypothesized, on the basis of previous studies conducted in northern mixed-grass regions of the Great Plains (10,12), that prairie dog colonies in the southern shortgrass prairie would support greater species richness than would the surrounding prairie. We also hypothesized that species richness on the edge of colonies would be greater than in the interior of colonies. Species richness should be greater where two habitat types, i.e., prairie and colony adjoin (13). According to disturbance-diversity theory, the two disturbances of prairie dogs and cattle grazing at colony edges should result in greater species richness than either disturbance alone (14,15). Thus, our objectives were to determine whether plant species composition and richness varied within and among prairie dog colonies and to determine whether plant species composition varied between the prairie dog colonies and surrounding grasslands.
| Introduction | Materials and Methods | Results | Discussion | References | Top of Page | Table of Contents | Home |
During a 1 wk period in May 1996, we studied plant communities on eight prairie dog colonies ranging in size from 3 to 302 ha in Cimarron County, Oklahoma. Cattle had grazed the area for several years (land owners, pers. comm.), but no data were collected to compare the differences in grazing pressure between sites. The study sites were located in the southwestern portion of the Great Plains Steppe Province (16). Cropland and shortgrass prairie compose most of Cimarron County (17). The vegetation is native shortgrass dominated by buffalo grass (Buchloe dactyloides), hairy grama (Bouteloua hirsuta), perennial three-awn (Aristida sp.), sideoats grama (B. curti-pendula), plains yucca (Yucca glauca), plains prickly pear (Opuntia macrorhiza), and broom snakeweed (Gutierrezia sarothrae). Annual rainfall ranges from 380 to 890 mm, mean annual temperature ranges from 10 to 15°C, and wind velocity ranges from 10 km/hr at 0800 hr to 26 km/hr at 1500 hr (17,18). Precipitation during the spring of 1996 was below normal (19) and the vegetation was in a state of drought-induced dormancy.
We mapped the extent of eight prairie dog colonies on 7.5 min United States Geological Survey (USGS) topographic maps and digitized their boundaries using Sigma Scan v. 3.9 (20) to estimate the area of each colony. We established a transect along the long axis of each colony extending from 20 m outside to its center. The transect began at the northern edge if the long axis was predominantly north to south and at the western edge if it was predominantly east to west. Contiguous 1 m2 quadrats were sampled along each transect from 20 m outside to colony edge (prairie) and from the colony edge to 20 m inside the colony (edge). We sampled the interior by placing a quadrat every 10 m to 80 m, and then every 20 m to the center of the colony. Thus, the number of interior quadrats varied among colonies (13, 7, 7, 5, 46, 26, 9, and 13) because of the large differences in size (3-302 ha) among them. We estimated cover for each species in each quadrat according to a modified 9-class Braun-Blanquet (21) cover-abundance scale (trace, <1%, 1-2%, 2-5%, 5-10%, 10-25%, 25-50%, 50-75%, 75-100%). Voucher specimens are deposited in the Oklahoma State University Herbarium.
Initial inspection of the quadrat data revealed relatively few species per square meter and substantial fluctuation in species composition between adjacent quadrats. To reduce noise in the data, we combined all of
{Page 13}
the aforementioned prairie quadrats, edge quadrats, and interior quadrats by site. Thus, there were three samples for each site. An additional reason for combining the quadrats was that we could not consider individual quadrats to be true replicates (22,23)
We used Detrended Correspondence Analysis (DCA) on the composite samples (prairie, edge, and interior as described above) to examine patterns in species composition within and among study sites. DCA is a robust technique based on reciprocal averaging (24-27) and can be useful in assessing gradients in species composition. Analyses were performed by using the program CANOCO (28), with a downweighting of rare species (i.e., species with frequencies less than 20% of the maximum) and square-root transformed species cover value. These options decrease the disproportionate effect of infrequent species and generally enhance interpretation (29).
Although DCA is useful for describing and displaying trends in species composition, it is primarily used as an exploratory technique, and hence, it is not associated with inferential statistics such as testing significance. Therefore, we performed a partial Canonical Correspondence Analysis (pCCA) with a Monte Carlo test to assess effects of location within a site on species composition (28). Because the beta diversity was low in the original data set, a linear Redundancy Analysis (RDA), followed by a Monte Carlo test, was performed to assess the effects of prairie, edge, and interior within sites (30).
| Introduction | Materials and Methods | Results | Discussion | References | Top of Page | Table of Contents | Home |
Forty-two plant species representing 13 families were present on the eight colonies and adjacent prairie. Thirty-two species were found within prairie dog colonies. Grasses and composites had the most species represented, 9 and 11, respectively. The most frequently observed species in all three sample types, i.e., prairie, edge, and interior, were buffalo grass, broom snakeweed, and globe mallow (Sphaeralcea coccinea). The most widely distributed species, found in at least 75% of the colonies, included plains prickly pear, broom snakeweed, globe mallow, perennial three-awn, buffalo grass, and blue grama (Table 1).
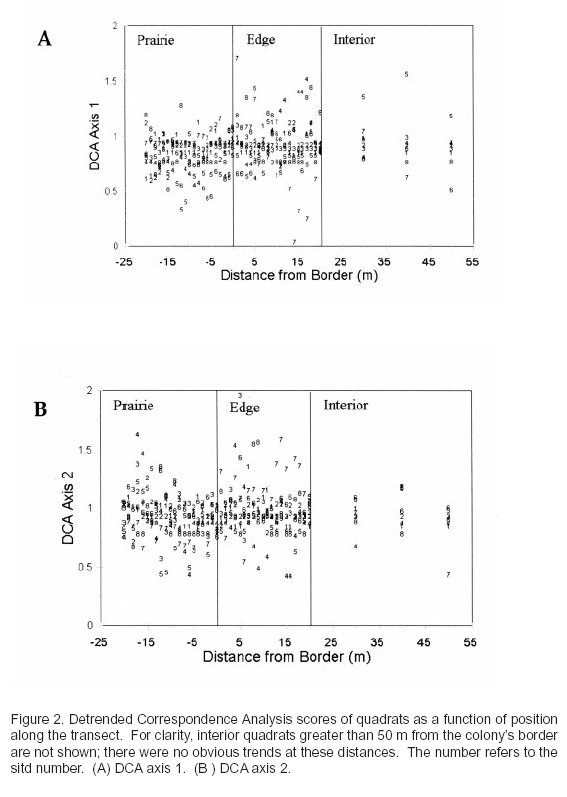
A DCA (Fig. 1A) revealed that the major gradients in species composition were primarily related to variation among sites, rather than variation within sites. Sites with low first axis scores, e.g., Sites 3 and 4, had relatively high cover of sideoats grama, and sites with high first axis scores, e.g., Sites 6 and 7, had a high cover of perennial three-awn and other grama species, and therefore might represent a midgrass to shortgrass gradient (Fig. 1B). The relative locations of the habitat types, i.e., prairie, edge, interior, in ordination space were not consistent among sites, which suggests composition does not vary consistently among these three habitats. Hence, we performed a pCCA with site locations as dummy covariables and the site types as dummy explanatory variables. A Monte Carlo test revealed that location within a site had no effect on species composition (P > 0.05). The beta diversity was low in the original data set (only 1.6 SD units along the first axis; Fig. 1). However, an RDA followed by Monte Carlo tests resulted in no significant effects of edge, prairie, and interior within sites. It is possible that the lack of interpretable or significant effects occurred because of the arbitrary cut-off of 20 m to represent the edge conditions. For example, true edge may only extend a few meters into the colony. To assess this possibility, we performed a DCA on quadrats, which revealed no obvious patterns in first axis and second axis scores as a function of distance along the transect (Fig. 2).
| Introduction | Materials and Methods | Results | Discussion | References | Top of Page | Table of Contents | Home |
On our study sites plant species richness was less than values reported from other studies conducted on shortgrass and mixed-grass prairie. Bonham and Lewick (10) found 35 species inside a colony during a study conducted on a shortgrass prairie in Colorado. Uresk (12) identified 39 species associated with colonies in a South Dakota mixed-grass prairie. Archer and co-workers (31) and Coppock and co-workers (32) reported greater species richness on prairie dog colonies than on surrounding mixed grass prai-
{Page 14}

{Page 15}

{Page 16}
{Page 17}
rie. We found higher species richness (r) on Sites 4 (r = 18), 5 (r = 15), 6 (r = 15), and 7 (r = 18) than on other colonies. Expanding or younger colonies may have greater species richness than non-expanding or older colonies (32). Although we do not know the ages of all of our colonies, Sites 4, 5, 6, and 7 had recently expanded, which created recently colonized conditions. Colony expansion at Sites 1 (r = 7) and 8 (r = 9) were hindered by poisoning of the prairie dogs. The colony at Site 2 (r = 7) was unable to expand because of topography, and Site 3 (r = 12) was severely overgrazed by cattle.
One possible explanation for the greater species richness of vegetation on prairie dog colonies of northern mixed prairie and shortgrass prairies to the north of our study area is that these more northerly grasslands have a more equal mix of plants representing the C3 and C4 photosynthetic groups. Vegetation dominated by C4 grasses occupies most of the southern portion of the Great Plains grasslands, whereas the more northerly portions are codominated by C4 and C3 grasses (33). Hence, disturbance by prairie dogs in the C3-C4 grass codominated grasslands may have greater potential for richness. Conversely, the pattern of richness we see in our study and other studies may be an artifact of other unidentified factors. For example, several kinds of disturbances and disturbance interactions, e.g., fire and grazing interactions, herbivore-disturbance interactions, can also alter plant community dynamics (33).
King (34) and Koford (35) concluded, based on plant height and species composition differences between colonies and surrounding prairie, that vegetation was the key factor in separating colonies from surrounding prairie. It is possible that the more ephemeral species, which may show preferences for prairie, edge, or interior conditions, were not present on our sites because of drought. Such species may have provided more additional experimental power to discriminate between species composition among microsites, i.e., prairie, edge, interior. Koford (35) found more plant species, especially forbs, within colonies than on surrounding shortgrass prairie in Colorado. Forbs, which were rare in our study because of drought, are minor components of biomass, but can contribute significantly to plant species richness (14). Yet their presence is dependent largely on local biotic factors (33).
Species composition differed more among sites than within sites in our study, but plant species richness has been reported to vary within prairie dog colonies (1). Although prairie dog activities profoundly alter the physical structure of the vegetation, we found that location within a colony (prairie, edge, and interior) had no significant effect on species composition. Greater heterogeneity of plant cover within prairie dog colonies compared to surrounding prairie has been reported for prairie dog colonies within mixed-grass prairies (36-38). Although our prairie quadrats may have exhibited some characteristics of edge habitats, we still expected to find differences between the interior and edge habitats within each colony. Drought conditions (9) and/or heavy cattle grazing may have negatively affected the results of our study by preventing growth of some species. That no difference in plant richness occurred within colonies may have implications for animals reported to be dependent on prairie dog colonies (8) including the mountain plover (Charadrius montanus), burrowing owl (Athene cunicularia), swift fox (Vulpes velox), and northern grasshopper mouse (Ony-chomys leucogaster). Our study suggests that there may be differences between the northern and southern regions of the Great Plains. The activities of prairie dogs may alter mixed-grass and shortgrass prairie differently. In general, herbivory has little effect on richness in shortgrass prairie (39). This generality may not extend into mixed-grass prairie regions.
In conclusion, we found substantial variation in plant species composition among prairie dog colonies, but no consistent patterns within colonies. We also found no difference in vegetation composition between prairie dog colonies and surrounding prairie. Factors such as variation in precipitation, topoedaphic factors, extent of historical ungulate grazing, and current grazing pressures may confound the influence of prairie dogs on vegetation. Controversy surrounds federal listing of prairie dogs,
{Page 18}
studies on prairie dog colonies are inconsistent in methodology, and drought and cattle grazing are commonplace disturbances in Great Plains grasslands. Hence, additional information is needed to make informed conservation and management decisions concerning prairie dog colonies in the southern Great Plains. This indicates the need for a network of studies similar to ours to be conducted throughout the southern Great Plains, but using larger spatial scales for sampling than we used.
We thank the Oklahoma Cooperative Fish and Wildlife Research Unit (Oklahoma State University, Oklahoma Department of Wildlife Conservation, U.S. National Biological Service, and the Wildlife Management Institute, cooperating) for providing funding and logistic support for this project, the University of New Orleans and Southern Illinois University for providing technical support, J. Shaw for providing insightful discussions, and the land owners in Cimarron County who allowed us to use their land for this study. We also thank G. Feldhamer, E. Middleton, D. Wester, and anonymous POAS reviewers for providing comments that improved this manuscript.
| Introduction | Materials and Methods | Results | Discussion | References | Top of Page | Table of Contents | Home |
1. Whicker AD, Detling JK. Ecological consequences of prairie dog disturbances. Bioscience 1988; 38:257-270.
2. Clippinger NW. Habitat suitability index models: black-tailed prairie dog. US Fish Wildl Serv Biol Rep 1989; 82:1-21.
3. Hoogland JL. The black-tailed prairie dog. Chicago (IL): University of Chicago Press; 1995.
4. Merriam CH. The black-tailed prairie dog of the Great Plains In: US Department of Agriculture Yearbook for 1901. Washington D.C.: US Government Printing Office; 1902.
5. Miller B, Ceballos G, Reading R. The prairie dog and biotic diversity. Conserv Biol 1994;8:677-681.
6. Cully JF. Plague, prairie dogs and blackfooted ferrets. In: Oldemeyer JL, Biggins DE, Miller BJ, Crete R, editors. Management of prairie dog complexes for the reintroduction of the black-footed ferret. US Fish Wildl Serv Biol Rep. 13; 1993. p 38-48.
7. Benedict RA, Freeman PW, Genoways HH. Prairie legacies-mammals. In Samson FB, Knopf FL, editors. Prairie conservation: preserving North Amer-ica's most endangered ecosystem. Washington, D.C.: Island Press; 1996. p 149-166
8. Kotliar NB, Baker BW, Whicker AD, Plumb G. A critical review of assumptions about the prairie dog as a keystone species. Environ Manag 1998; 24:177-192.
9. Weltzin JF, Dowhower SL, Heitschmidt RK. Prairie dog effects on plant community structure in southern mixed-grass prairie. Southwest Nat 1997; 42:251-258.
10. Bonham CP, Lewick A. Vegetation changes induced by prairie dogs on shortgrass range. J Range Manage 1976; 29:221- 225.
11. Winter SL, Cully JF Jr, Pontius JS. Influence of prairie dog colonies on vegetation communities in Kansas shortgrass prairie. In Thorpe J, Steeves T, Gollop M, editors. Proc Fifth Prairie Conservation and Endangered Species Conference, Provincial Museum of Alberta, Edmonton; 1999.
12. Uresk DW. Black-tailed prairie dog food habits and forage relationships in western South Dakota. J Range Manage 1984;37:325-329.
13. Meffee GK, Carroll CR. Principles of conservation biology. Sunderland (MA): Sinauer Associates Inc; 1994.
14. Collins SL, Glenn SM. Effects of organismal and distance scaling on analysis of species distribution and abundance. Ecol Appl 1997;7:543-551.
15. Connell JH. Diversity of tropical rain forests and coral reefs. Science 1978;199:1302-1310.
16. Bailey RG. Description of the ecoreg-ions of the United States. Washington D.C.: USDA Forest Serv Misc Publ 1391; 1995.
{Page 19}
17. Murphy RS, Abernathy EJ, Allgood FP, Worth PR, Meinders HC, Wammah LW, Icenhower JC. Soil survey of Cimarron County, Oklahoma. Washington D.C.: US Govt Printing Office; 1960.
18. Austin ME. Land resource regions and major land resource areas in the U.S. (Exclusive of Alaska and Hawaii). US Department of Agriculture Handbook No. 296; 1965.
19. Barko VA, Shaw JH, Leslie DM Jr. Birds associated with black-tailed prairie dog colonies in southern shortgrass prairie. Southwest Nat 1999;44:485-490.
20. Sigma Scan [computer program] v. 3.6. Chicago (IL): SPSS Inc; 1993.
21. Braun-Blanquet J. Plant sociology: the study of plant communities. Fuller CD, Conrad HS, translators and editors. London: 1932.
22. Hurlbert SH. Pseudoreplication and the design of field experiments. Ecol Monogr 1984;54:187-211.
23. Heffner RA, Butler MJ, Reilly CK. Pseudoreplication revisited. Ecology 1996; 2558-2562.
24. Gauch HG Jr. Multivariate analysis in community ecology. Cambridge (MA): Cambridge University Press; 1982.
25. Peet RK, Knox RG, Case JS, Allen RB. Putting things in order: the advantages of detrended correspondence analysis. Am Nat 1998;131:924-934.
26. Hill MO. DECORANA-a FORTRAN Program for Detrended Correspondence Analysis and Reciprocal Averaging. Ithaca (NY): Cornell University; 1979.
27. Hill MO, Gauch HG Jr. Detrended Correspondence Analysis, an improved ordination technique. Vegetatio 1980; 42:47-48.
28. CANOCO [computer program] v. 2.1. Wageningen: TNO Institute of Applied Computer Science; 1987.
29. CANOCO for windows [computer program]. Ithaca (NY): Microcomputer Power; 1998.
30. ter Braak CJF, Prentice IC. A theory of gradient analysis. Adv Ecol Res 1988; 18:271-317.
31. Archer S, Garrett MG, Detling JK. Rates of vegetation associated with prairie dog (Cynomys ludovicianus) grazing in North American mixed-grass prairie. Vegetatio 1987;72:159-166.
32. Coppock DL, Detling JK, Ellis JE, Dyer MI. Plant-herbivore interactions in a North America mixed-grass prairie: I. Effects of black-tailed prairie dogs on interseasonal above ground biomass and nutrient dynamics and plant species diversity. Oecologia 1983;56:1-9.
33. Paruelo JM, Lauenroth WK. Relative abundances of plants functional types in grasslands and shrublands of North America. Ecol Appl 1996;6:1212-1224.
34. King JA. Social behavior, social organization, and population dynamics in a black-tailed prairie dog town in the Black Hills of South Dakota. Ann Arbor (MI): Contribution of the Vertebrate Laboratory No. 67; 1955.
35. Koford CB. Prairie dogs, whitefaces, and blue grama. Wildl Monog 3; 1958.
36. Agnew W, Uresk DW, Hansen RM. Flora and fauna associated with prairie dog colonies and adjacent ungrazed mixed-grass prairie in western South Dakota. J Range Manage 1986;39:135-139.
37. Cincotta RP, Uresk DW, Hansen RM. Demography of black-tailed prairie dog populations reoccupying sites treated with rodenticide. Great Basin Nat 1987;47:339-343.
38. Sharps J, Uresk D. Ecological review of black-tailed prairie dogs and associated species in western South Dakota. Great Basin Nat 1990;50:339-345.
39. Milchunas DG, Lauenroth WK, Burke IC. Livestock grazing: animal and plant biodiversity of shortgrass steppe and the relationship to ecosystem function. Oikos 1998;83:65-74.
40. Kartez JT. A synonymized checklist of the vascular flora of the United States, Canada, and Greenland. 2nd edition. Portland (OR): Timber Press; 1994.
Received: November 30, 2000; Accepted: April 12, 2001