
|
 |
|
{Page 53}
Department of Sociology, Anthropology and Social Work, Texas Tech University, Lubbock, TX 79409
Department of Biology, University of Central Oklahoma, Edmond, OK 73034
Department of Biology, University of North Carolina at Asheville, Asheville, NC 28787
Department of Biology, University of Central Oklahoma, Edmond, OK 73034
Acute Diseases Division, Oklahoma State Department of Health, Oklahoma City, OK 73117
Arthropod-Borne and Infectious Diseases Laboratory, Department of Microbiology, Foothills Campus, Colorado State University, Fort Collins, CO 80523
We conducted a statewide survey of Oklahoma small mammals to test for antibodies against rodent-borne viral diseases. Four rodent species had antibody to Sin Nombre virus (SNV), the primary causative agent of hantavirus pulmonary syndrome (HPS), and two species had antibody to Whitewater Arroyo virus, an arenavirus associated with human fatalities. The rodent reservoirs for other HPS-causing North American hantaviruses also occur within the state. This 13-month seroepidemiologic survey assayed 26 species of small mammals belonging to 14 genera collected from 14 locales in 8 of the 9 major physiognomic regions of Oklahoma. Of 686 captures, 5.25% were SNV-seropositive. The SNV-seropositive species were Peromyscus maniculatus, P. gossypinus, P. leucopus, and Sigmodon hispidus. Of the peromyscines, 8.4% of captures were SNV-seropositive. Four localities exhibited two or more seropositive species. We found high capture and SNV-seropositive rates for the white-footed mouse, P. leucopus: 25% of all captures, 67.7% of all peromyscine captures, and 44.4% of all seropositive individuals. P. leucopus has been associated with HPS cases caused by New York hantavirus and by Monongahela hantavirus in Pennsylvania. Health professionals and those at risk for occupational exposure should be aware that pathogenic rodent-borne viruses occur within the state. © 2001 Oklahoma Academy of Science1
1Note added in press: The first fatal human hantavirus case in Oklahoma (Texas County) was confirmed by the Centers for Disease Control in late November 2001.
| Introduction | Material and Methods | Results | Discussion | References | Top of Page | Table of Contents | Home |
Sin Nombre virus (SNV) is the primary etiological agent of hantavirus pulmonary syndrome (HPS) in North America (1,2). From the outset of the 1993 outbreak in the Four Corners region of the Southwestern United States through April 16, 2001, the Centers for Disease Control and Prevention (CDC) has reported 283 cases of HPS, with a human case fatality rate of 38% (3). The incidence of HPS remains highest in the Four Corners states. However, since January 1, 1994, there have been 203 cases in 31 states, with a case fatality rate of 30%. The only known HPS case (non-fatal) in Oklahoma occurred in the
{Page 54}
fall of 1996 (4). This person lived in Texas County in the northwest Panhandle region of the state and apparently was exposed while working in the cab of an abandoned truck infested with rodent nests. The six states contiguous to Oklahoma have recorded numerous HPS cases (3): New Mexico, 41; Colorado, 25; Kansas, 14; Texas, 13; Arkansas, 0; Missouri, 0; and nearby Louisiana has recorded a single case.
Present evidence suggests that coevolution of rodent reservoir hosts and hanta-viral species has resulted in host specificity (2, 5, 6). Eleven North American hantaviruses have been described (1, 2). Five of these have been associated with HPS (1): SNV (primary reservoir host is the deer mouse, Peromyscus maniculatus); Monon-gahela (associated with the white-footed mouse, P. leucopus); New York virus (NY; primary reservoir host is the white-footed mouse, P. leucopus); Black Creek Canal virus (BCC; primary host is the cotton rat, Sig-modon hispidus); and Bayou virus (BAY; primary host is the rice rat, Oryzomys palustris). Other North American hantaviruses, all of unknown pathogenicity, have been associated with various rodent reservoirs: Mule-shoe (MULE; S. hispidus); El Moro Canyon (ELMC; the western harvest mouse, Reithrodontomys megalotis); Monongahela (which has also been associated with the deer mouse); Blue River (white-footed mouse); Prospect Hill (PH; meadow vole, Microtus pennsylvanicus); Isla Vista (ISLA; California vole, Microtus californicus); and Bloodland Lake (prairie vole, M. ochragaster).
With respect to biogeography, Oklahoma is one of the most diverse states, on the basis of land forms and relative land area, in the continental United States. Variation in topography and geology, elevation, botany, and precipitation results in nine to twelve ecological biozones, or physiognomic regions depending on author (7, 8). These biozones vary from the high-elevation piñon-juniper mesas of the northwest Panhandle to the low-elevation cypress-oak swamplands in the far southeast, from the mesquite savannas of the extreme southwest to the hardwood forests of the Ozark Plateau in the northeast. These natural communities contain an abundance of rodent species, with the diversity, and density of some species, being greater in the grassland habitats in the western half of the state. Of those sigmodontine rodents listed above which are associated with hantaviruses, all occur within Oklahoma. Furthermore, Oklahoma not only contains the western-most popula-

{Page 55}
tions of some eastern rodent species and the eastern-most populations of some western rodent species but also many southern rodent species have expanded their ranges northward in Oklahoma during the recent historic past (7). The purpose of this study was to conduct a survey of rodents for the presence of antibody to SNV and to Whitewater Arroyo virus (WWAV), a New World arenavirus, given Oklahoma's physiognomic diversity, the large number of rodent species occurring within the state that have been associated elsewhere with North American hantaviruses, and the higher occurrence of HPS in most adjoining states.
| Introduction | Material and Methods | Results | Discussion | References | Top of Page | Table of Contents | Home |
The preponderance of HPS cases has been reported primarily from areas of relatively high elevation and is associated with grassland habitats exhibiting relatively high diversity and density of rodent species. The key objective of this study was to sample rodent populations from the state's nine major physiognomic regions (Fig. 1) to determine the potential risk of HPS across Oklahoma. Possible study locales at state parks, wildlife management areas, nature conservancy areas, and university research stations/field sites in each biozone were identified and permissions to work there acquired. Small mammal collecting permits were obtained from the Oklahoma Department of Wildlife Conservation. Trapping was conducted on an opportunistic basis with the goal of one trapping session per month beginning in July 1998 and ending in July of 1999.
Field Collections: Field collection protocols for rodents were approved by the University of Oklahoma Health Sciences Center (OUHSC) Institutional Animal Care and Use Committee. The majority of specimens were live-captured using Sherman 3x3.5x9" folding galvanized aluminum traps. Snap traps were used initially to collect voucher specimens and for endoparasitological studies of co-morbidity. Because this project was designed as a preliminary statewide survey, a mark-release-recapture method of determining population sizes (9) was not used. A trapping locale was defined as a spatially coherent constellation of trapping sites. At each study locale, trapping was conducted in a variety of habitat types, designated as sites, distinguished by differing botanical assemblages. The term locality is used to refer to a locale that was trapped on several occasions but each time composed of different trapping sites or to an aggregate of locales with an essentially common botanical assemblage. Most trap sites occurred along roadsides, in grazed areas, in wildlife management areas and state parks, and were considered to be disturbed communities. At each trap site, botanical specimens were collected and keyed to species. These plant specimens are curated, and a list of plant taxa for each site is on file, at the University of Central Oklahoma. Traps were set in late afternoon or early evening, baited with rolled oats, and collected early the following morning for processing of captured animals. Trapping was carried out on successive nights over a 2-4 d period. During cold weather, cotton balls were put in the traps for use as insulation by trapped rodents. Pairs of traps were spaced approximately 10 m apart along grid lines approximately 200 m long. From 250-600 traps were set each night at the various locales, depending on the number of sites per locale. Initially, all captures were released at the original capture site, but as the study progressed most of the captures were sacrificed using the accepted technique of cervical dislocation (10-12). The sacrificed specimens were frozen and stored at the OUHSC for the duration of the field project and now have been transferred to the Natural Science Research Laboratory at the Museum of Texas Tech University. Standardized procedures regarding humane capture, processing, and bleeding as well as human safety precautions were employed (see 2, 11). Data collected for each specimen included species, age (adult, subadult), sex, morphometrics (ear length, tail length, etc.), and reproductive status (scrotal, pregnant, lactating, etc.). Captured rodents were bled via the retro-orbital sinus. Handling and orbital sinus bleeding are not associated negatively with survival for peromyscines (13). Several drops of blood, about 0.5-1.0 mL, were collected into cryovials by using heparinized hematocrit-
{Page 56}
capillary tubes. Blood specimens were stored on wet ice while processing animals in the field and on wet or dry ice during transportation until they could be curated in cold storage facilities at the OUHSC.
Laboratory Procedures: Blood specimens were shipped to the Arthropod-Borne and Infectious Diseases Laboratory at Colorado State University where enzyme-linked immunosorbent assays (ELISA) were conducted as described by Mills and co-workers (2). Because antibodies to other North American hantaviruses are cross-reactive with SNV antigen, ELISA detects but does not distinguish infections caused by closely related hantaviruses (2). Blood samples were screened at 1:100 for IgG antibody to SNV and by immunofluorescence for antibody to WWAV, an arenavirus found in North American woodrats (14) and of interest because of the pathogenicity of many South American arenaviruses and the recent association of WWAV with human fatalities in California (discussed below). Antibody- positive samples were titrated to determine end points.
| Introduction | Material and Methods | Results | Discussion | References | Top of Page | Table of Contents | Home |
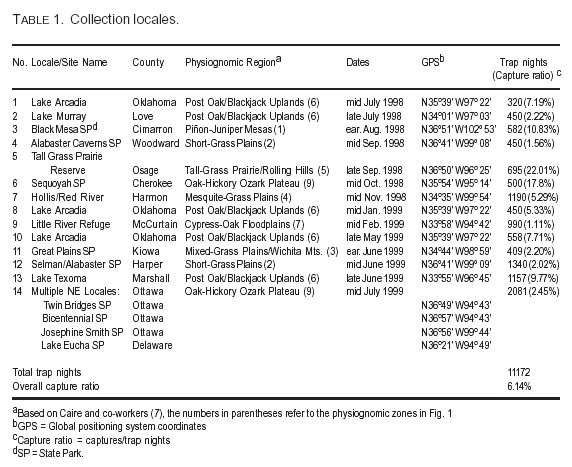
During the 13-month study period, the field team sampled small mammal populations at 14 distinct collection locales in eight of the nine physiognomic regions (Fig. 1). Lake Arcadia was sampled on three separate occasions because of the high hantavirus activity found there from the project's inception (52.8% of all SNV seropositives during the study). At the Lake Arcadia locales, different sites were sampled on each trip. Multiple locales in the Ozark Plateau region were sampled in the extreme northeastern part of the state and are referred to as the "NE Oklahoma" locality in Table 1. This table lists the locales, county, physiognomic zone, collection dates, GPS coordinates, and trap nights/capture ratio for each locale. As noted above, within each locale trapping was conducted at several sites, with varying degrees of anthropogenic disturbance.
{Page 57}
Trap nights for the 13-month study totaled 11,172 with a capture:trap nights ratio of 6.1%.
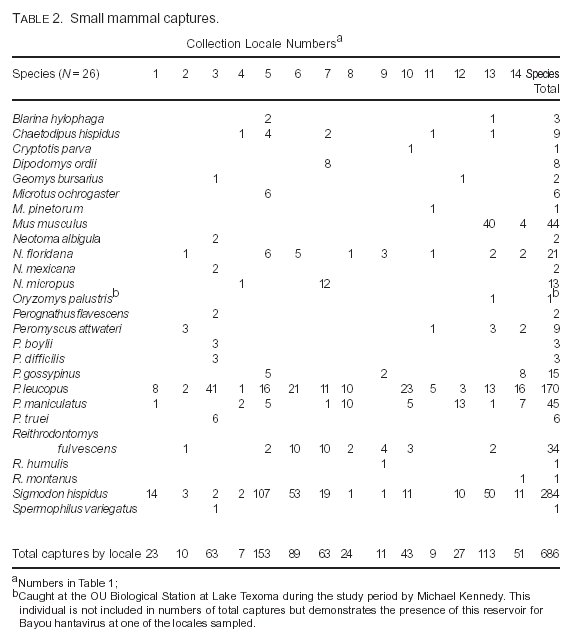
Table 2 lists the 686 small mammal captures by locale and species. We trapped 26 small mammal species representing 14 genera during the study period. The vast majority of captures belonged to Neotoma spp., Peromyscus spp. and S. hispidus. At one highly disturbed site, 40 specimens of Mus musculus were captured. In fact, the sig-modontine genera Peromyscus and Sigmodon accounted for more than three-quarters of all captures, with about two-thirds of all captures from two species, the white-footed mouse (25%) and the cotton rat (41%). Whereas only about 25% of the species captured yielded ample sample sizes (N > 20), the coverage was broad for these rodent genera occurring within Oklahoma and which have been associated in adjacent states with hantaviruses and arenaviruses: (a) all four Neotoma spp.; (b) seven of eight Peromyscus spp.; (c) three of four Reithrodontomys spp.; and (d) both Microtus spp. During the study period, no specimens of Ochrotomys, Baiomys, or Onychomys were captured although these genera are found in the state.
{Page 58}
Of the 686 total captures, 36 (5.25%) were seropositive for SNV. These SNV-seropositive specimens were captured at five different localities (Fig. 1). At locales with SNV-seropositives, the prevalence for a species varied from 4% to 50% (although the sample size was small at many locales). Table 3 lists the seropositives by locale for both SNV and WWAV. Four species had antibody which is reactive to SNV antigen (Table 4): (a) the cotton mouse, P. gossypinus (13.3% of all captures seropositive); (b) the white-footed mouse (8.2% seropositive); (c) the deer mouse (11.1% seropositive); and (d) the cotton rat (5.3%). Of the five different localities with seropositive rodents, four had two or more seropositive species, and the Tall Grass Prairie Preserve had all four seropositive species. White-footed mice and cotton rats accounted for 66% of all captures, and both of these species were trapped at 13 of 14 locales. Seropositives from these two species occurred at five locales each, and together they accounted for 80.6% of all SNV- seropositives.
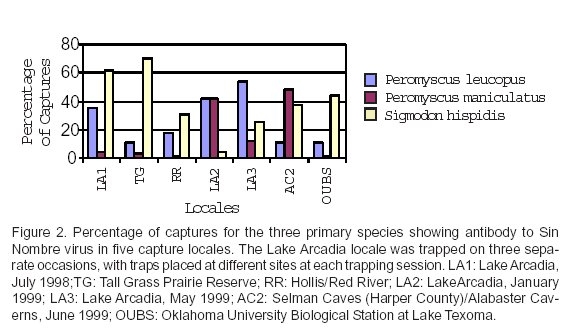
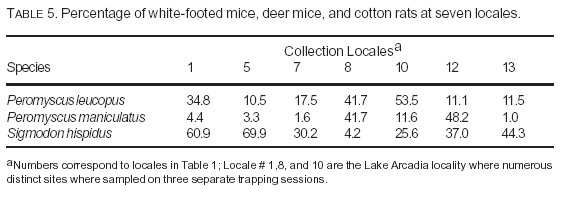
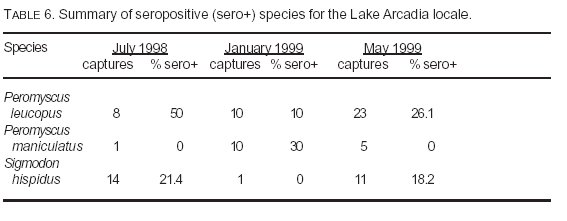
Table 5 lists the percentage of all captures for the three principal seropositive species that accounted for 94% of all sero-positives (white-footed mice, deer mice, cotton rats) at locales with seropositive individuals. Figure 2 is a graphic depiction of this information. Of these three seropositive species, the deer mouse was the least often captured at most locales. The only exceptions were Lake Arcadia (1/99) and Selman Caves (Harper County)/Alabaster Caverns (6/99). Table 6 summarizes the captures of these same three species at Lake Arcadia, the only locality sampled on multiple occasions during the study period. Although the numbers were low during the January trapping session, both the highest capture ratio and the highest percentage seropositive for the deer mouse occurred during that month.
Overall, Peromyscus spp. accounted for 58.3% (N = 21) of all SNV-seropositives. Of these, 90.5% were adults, and 85.7% were males. The cotton rat accounted for 41.7% (N = 15) of SNV-seropositives; all seroposi-tives were adults, and 46.7% were males.
| Introduction | Material and Methods | Results | Discussion | References | Top of Page | Table of Contents | Home |
Capture Success: Capture success varied by collection locale, from 1.1% to 22% (Table 1). Although many factors such as natural cycles in rodent populations affect capture success, during the two summers when trapping was carried out in this study, most of the state suffered from an exceptional drought and heat wave. Drickamer and co-workers (15) conducted an experimental study that uncovered both extrinsic and intrinsic factors affecting trap-response hetero-
{Page 59}

geneity for the adult house mouse, Mus musculus. Specifically, humidity, temperature, and animal's sex affected trapping response. Lewellen and Vessey (16) also conducted an experimental study that suggested Sherman live traps do not trap representative populations, especially in winter, and may result in undercounts for Peromyscus sp. In our Oklahoma study, of the four sites with the highest capture success (Black Mesa State Park [SP]—10.8%; Tall Grass Prairie Reserve—22%; Sequoyah SP—17.8%; and OU Biological Station—9.8%), usually only a single species predominated. At Black Mesa SP, the white-footed mouse accounted for 65.1% of all captures; at the Tall Grass Prairie Preserve and Sequoyah SP, the cotton rat accounted for 69.9% and 59.6% of captures, respectively; at the OU Biological Station, cotton rats and house mice together accounted for 79.7% of all captures (Table 2).
Most studies have reported high overall capture numbers for adult males (17). This has been attributed to the larger home ranges for males. For sigmodontines (75% of all captures in this study), more males (53%) than females were captured. However, this result is largely due to the Peromyscus spp. with 57% of captures being males (N = 132) versus 49% of cotton rats being males (N = 139). These differences are not significant (X 2 = 3.64, 1 df, P = 0.056) for male versus female captures of sigmodontines.
Most previous studies also have shown relatively high SNV-seropositive rates for the western harvest mouse, R. megalotis (5, 17, 18, 19,). This species is associated with ELMC (20), and antibody to ELMC is cross-reactive to SNV. In Oklahoma, the western harvest
{Page 60}

mouse occurs only in the northwestern Panhandle. Three of the four Reithrodontomys spp. occurring in Oklahoma were captured in this study, but no western harvest mice individuals were caught during trapping in the Panhandle region.
Many species of the piñon-juniper peromyscine guild were not captured in high numbers, but we only trapped on a single occasion in that locality. Given the seropositive rates reported elsewhere (5, 21-23) for the brush mouse (P. boylii) and the piñon mouse (P. truei), these two species are of great interest. Similarly, the rice rat (O. palustris) is also of great interest given its association with the HPS-causing BAY virus (24, 25) in Texas and Louisiana. The rice rat occurs in southern, eastern, and northeastern Oklahoma, apparently expanding its range in recent decades, although it does not appear to occur in high numbers. During this study, a single specimen was captured by Michael Kennedy at the OU Biological Station at Lake Texhoma. This specimen has been submitted for testing.
Capture Locales: All of the trapping sites in our study occurred in what we considered to be disturbed habitat (roadside ditches, state parks, wildlife management areas, wheat/grain fields, grazed ranchland, etc.).
{Page 61}
It is difficult to find areas in Oklahoma that are not disturbed to one degree or another. Boone and co-workers (26) found that seronegative rodents tended to occur in habitats characterized by low elevation, level topography, and low vegetation productivity and species variability. Bennett and co-workers (17) found, based on trapping records for the period 1984-1992 in Orange and San Diego Counties of southern California, that deer mice and western harvest mice were collected more often in disturbed (ruderal) habitats dominated by introduced weeds and ornamental shrubs, grassland and sage-scrub habitats, or grassland/sage-scrub/chaparral ecotones. M'Closkey (27) found that deer mice and western harvest mice preferred disturbed, early succession habitats or open, patchy habitats with low shrub heights. Bennett and co-workers (17) found that, in Los Angeles County, the deer mouse was associated with sparse vegetation and boulder deposits.
SNV Seropositivity: SNV-seropositive specimens were collected at five distinct localities in this study. Four of these localities had at least two seropositive species, and the Tall Grass Prairie Preserve had all four species shown to have antibody during the study period. The 36 seropositive individuals (Table 4) in this study are from species previously found to be seropositive in other studies: white-footed mice, cotton mice, cotton rats and deer mice.
The white-footed mouse is associated with the HPS-causing NY hantavirus in the northeastern United States (28) and with HPS-causing Monongahela hantavirus in Pennsylvania (29). This species also has been found to be seropositive in North Carolina (30). At Molina in western Colorado, one of two white-footed mice captured was seropositive (22). Glass and co-workers (31) collected and tested 1500 small mammals in Florida, reporting a single cotton mouse and 123 cotton rats with antibody to SNV. The cotton rats also had antibody to BCC virus.
Deer mice usually are the most common species with relatively high seropositive rates and therefore are considered the principal vertebrate host for this virus species. Jay and co-workers (19) reported seroposi-tives for five peromyscine species, with deer mice accounting for 42.2% of 4,549 captures and seroprevalence of 11.8% over a 20-yr period for certain locations in California and the Channel Islands. Otteson and co-workers (18) found that deer mice accounted for 50.2% of 1,867 total captures, with 12.5% seropositive in northeastern California and Nevada. Graham and Chomel (32) found about 13-15% seropositives for deer mice on the Channel Islands, with large variation among sites and island populations. For the period of 1988-1997, Bennett and co-workers (17) reported four seropositive sigmo-dontine species from Orange and San Diego Counties in southern California, with deer mice representing 40.1% of captures and 9.1% of the 948 deer mice being seropositive. At Fort Lewis and Molina in Western Colorado, deer mice accounted for 85.5% and 63.1%, respectively, of all captures, with 9.6% and 9.4% seropositive, respectively (22). Antibody prevalence in deer mice has been reported to increase with altitude (5, 19).
Previous studies have indicated that larger and older males are more likely to be seropositive (5,17,18,21-23,26,32). To many authorities, this evidence has suggested horizontal transmission of hantaviruses for several species (9,17,33), related perhaps to intraspecific encounters (22,34) due to overlapping home ranges. In this study of Oklahoma rodents, 85.7% (18 of 21) of the seropositive peromyscines were males, whereas 47% (7 of 15) of the S. hispidus seropositives were male. These results are significant for seropositive captures by sex for the two genera (X 2 = 6.29, 1 df, P = 0.012).
In comparison to previous reports on peromyscines, our study is unique in the capture frequency and seropositivity of white-footed mice vis-à-vis deer mice. Both species are geographically widespread in the continental United States. The former predominates in the Upper Midwest and eastern United States, whereas the latter predominates in the western United States. For example, in a comprehensive study of nine biotic communities and numerous sites in several southwestern states, Mills and co-workers (5) caught 3,069 small mammals, with 42,831 trap nights and a capture ratio of 7.2%, and found that deer mice were the most commonly captured species, with 31%
{Page 62}
of total captures. Weigler and co-workers (30) reported four seropositives from 301 Peromyscus spp. collected in North Carolina. Two of these were white-footed mice from a coastal island biome, and two were from a mountain biome where deer mice and white-footed mice were sympatric.
In our study, white-footed mice represented 25% of all captures, 67.7% of all peromyscine captures, and 44.4% of all seropositive individuals. Deer mice accounted for only 6.6% of all captures, 17.9% of all peromyscines captured, and 13.9% of all seropositives. In Oklahoma, the white-footed mouse shows distinctive pelage changes from east to west (7). Using micro-satellite markers, Schmidt (35) confirmed that two white-footed mouse cytotypes exist in Oklahoma and that the well-documented hybrid zone in central Oklahoma demonstrates introgression of the northwestern alleles into the southwestern genome. Morzunov and co-workers (36) discussed the geographic distribution of four mitochondrial DNA haplotypes of white-footed mice in relation to the hantavirus species borne by this mouse species. These authors also substantiated a stable hybrid zone in central Oklahoma among the northwestern and southwestern karyotypes. Mor-zunov and co-workers conducted phylogenetic analyses of white-footed mice and hantaviral species, including a specimen collected by the CDC from Murray County in 1993. (Immediately following the Four Corners outbreak, a study of rodents from several western states recorded a single SNV-seropositive white-footed mouse from Murray County in south-central Oklahoma [Centers for Disease Control and Prevention memo to J.M. Crutcher of the Oklahoma State Department of Health]). Morzunov and co-workers concluded that the NY virus borne by the white-footed mouse in the northeastern United States belonged to a monophyletic group that included the deer mouse-borne SNV and Monongahela viruses while the Oklahoma virus lineage, for which they proposed the name Blue River virus, formed an ancestral branch within the SNV clade with a virus borne by Indiana populations of white-footed mice.
The Lake Arcadia locality was an area exhibiting high rodent prevalence for SNV in this study. Focal areas with high hanta-virus prevalence, as well as cyclical patterns for seroprevalence, have been noted at several sites, for several rodent species, and for numerous hantaviruses (21,31,33). The Lake Arcadia locality accounted for more than half of all seropositives in this study, with individuals of three rodent species showing antibody to SNV. Otteson and co-workers (18) found that antibody prevalence among deer mice remained a constant 25-27% at one site over a multi-month period but that there were no seropositives at other sites over similar time periods. Bennett and co-workers (17) reported seropositive specimens in all months but noted a gradual increase in antibody prevalence in deer mice from December to June. Although the total number of rodents collected at Lake Arcadia was small (N = 90) and the study period was relatively short, deer mice captures at this locality fluctuated widely, with both higher capture frequencies and higher seropositivity in winter when seropositivity is least for the sympatric white-footed mice and cotton rats at this site.
Whitewater Arroyo Virus: The recent report (37, 38) of human fatalities in California associated with WWAV is of particular concern. In this study, we found two of the four woodrat species in the state seropositive for WWAV: the white-throated woodrat (Neo-toma albigula at Black Mesa SP) and the southern plains woodrat (N. micropus in Harmon County). Recently, based upon a longitudinal study from 1995-1999, Calisher and co-workers (39) reported that three woodrat species in southeastern Colorado (N. albigula, N. mexicana, and N. micropus) had antibody to WWAV. Fulhorst and co-workers (40) recently reported that WWAV is widely distributed throughout the western United States, including Oklahoma, in association with Neotoma rodents.
Conclusions: When the first Europeans traversed Oklahoma Territory from the eastern, forested mountains, they next encountered grasslands before entering a wide belt of timber that "crossed" the plains from north to south. Called the "crosstimbers" (8), the forests of this grassland-forest mosaic that bisected the state were dominated by short-
{Page 63}
post oak (Quercus stellata) and blackjack oak (Q. marilandica). Based on the 13-month survey reported here, zoonotic hantaviruses occur at the very least in rodents inhabiting the crosstimbers and western plains of Oklahoma (Fig. 1). Our sample sizes in the eastern part of the state did not permit an adequate appraisal of that region. Although most hantavirus studies cited above summarized longitudinal projects, the results of our preliminary survey do confirm many aspects of sigmodontine ecology as related to the natural history of SNV-associated hanta-viruses. The seroprevalence among Oklahoma peromyscines was 8.4%, which is similar to studies in the western United States, and to that reported for Kansas (41). Nevertheless, the presence of SNV-related hantaviruses as well as a Whitewater Arroyo-related arena-virus in Oklahoma needs to be confirmed by polymerase chain reaction (PCR) assays.
The salient findings of this study relate to the preponderance of white-footed mice both with regard to captures and to SNV-seroprevalence. Given the issues of host specificity (for both hantaviruses and arenaviruses), threshold density [density-dependent prevalence of infection (22)] and cross-species spillover into non-reservoir species (5), the results reported here offer preliminary insight into, and suggest promise for future studies of, the natural history of hantaviruses borne by the white-footed mouse, the deer mouse, and the cotton rat in Oklahoma. Because of the occurrence of the MULE virus in Texas cotton rats and the reported cocirculation of multiple hanta-virus species, it would be of interest to examine hantavirus epidemiology in this rodent species (42). The hybrid zones for white-footed mice present opportunities for investigating cross-species viral transmission, reassortment, or recombination of hantavi-ruses (43). Future studies also should focus on the natural history of the rice rat and its BAY virus to compare with those long-term studies of peromyscine-borne SNV in the western United States. As reported here and in several other studies (44), because SNV-seropositive rodents may be associated with anthropogenic disturbance, it is important to quantify habitat-structure attributes relevant to hantavirus ecology (45) and to test the hypothesis that ectoparasites potentially play a transmission role in the landscape epidemiology of hantaviruses (46). With regard to woodrats associated with WWAV (14), Caire and co-authors (7) have identified Oklahoma sites where the eastern woodrat (N. floridana), hybridizes with the western southern plains woodrat (N. micro-pus). A study at such sites could yield valuable information on the natural history of arenaviruses in the state.
For rodent-borne hantaviruses and arenaviruses, since there is no specific treatment available, developing effective prevention measures is dependent on knowledge of the ecology and epizootiology of the reservoir species (2). Detailed, longitudinal, ecologic surveillance studies can provide a better definition of potential disease-endemic areas than can a human-based surveillance system (9). Differences in SNV seroprevalence in rodent populations in different habitats have been documented and may help predict the variable disease risk to humans (2,9,47).
However desirable it may be to opera-tionalize an active rodent-borne virus surveillance system in the state, the fact remains that such programs are both expensive and labor-intensive.
A recent survey (48) of Oklahoma pest-control technicians found that whereas these workers were knowledgeable of an occupational risk for HPS and desired more information, they were less aware that HPS had occurred in Oklahoma and perceived hantaviruses primarily as a threat restricted to the southwestern United States. It is important for public health and primary health-care professionals, educators, field biologists, and others at risk of occupational as well as peridomestic exposure in Oklahoma to be aware of the presence of rodent-borne viruses across the state. The first HPS case in New England (Vermont) was reported in July, 2001 (49). Like most patients (75%) with documented exposure to rodents, this case appears to represent peridomestic exposure. Information on reservoir ecology, diagnosis, treatment, prevention, and control are readily accessible in electronic (e.g., CDC web site) or print (1,50) versions.
{Page 64}
This project was funded generously by the Presbyterian Health Foundation (Grant #1187) and the Oklahoma Center for the Advancement of Science and Technology (Project number HR98-016) to RA Nisbett, and was greatly facilitated by the Office of Research Administration at the University of Oklahoma Health Sciences Center (OUHSC). We especially acknowledge the many facilitative efforts of Dr. Frank Waxman, OUHSC Vice President for Research, and the Oklahoma Department of Wildlife Conservation. We also express our appreciation to the following: Oklahoma State Parks and Resorts, particularly Assistant Director Tom Crider as well as various park managers and rangers for their patience and cooperation, and the staff of the Tall Grass Prairie Preserve. The field collections benefited from the efforts and expertise of Sandy Stevens and Tamara Watts of the University of Central Oklahoma, and Kristi Bradley of the Oklahoma State Department of Health. We are grateful to the reviewers, and particularly to editors Clark Ovrebo and Ronald Tyrl, for constructive criticism, valuable advice and commendable patience.
| Introduction | Material and Methods | Results | Discussion | References | Top of Page | Table of Contents | Home |
1. Lee HW, Calisher CH, Schmaljohn C, editors. Manual of hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Seoul (Korea): WHO Collaborating Center for Virus Reference and Research (Hantavi-ruses), Asan Institute for Life Sciences, 1999. 250 p.
2. Mills JN, Yates TL, Ksiazek TG, Peters CJ, Childs JE. Long-term studies of hantavirus reservoir populations in the Southwestern United States: rationale, potential, and methods.Emerg Infect Dis 1999;5:95-101.
3. Centers for Disease Control and Prevention web site. <www.cdc.gov/ncidod/diseases/hanta/hps>; 2001.
4. Graves T, Crutcher JM. The first reported case of hantavirus pulmonary syndrome in Oklahoma. J Okla State Med Assoc 1998;91:327-330.
5. Mills JN, Ksiazek TG, Ellis BA, Rollin PE, Nichol ST, Yates TL, Gannon WL, Levy CE, Engelthaler DM, Davis T, Tanda DT, Frampton JW, Nichols CR, Peters CJ, Childs JE. Patterns of association with host and habitat: antibody reactive with Sin Nombre Virus in small mammals in the major biotic communities of the Southwestern United States. Am J Trop Med Hyg 1997;56:273-284.
6. Monroe MC, Morzunov SP, Johnson AM, Bowen MD, Artsob H, Yates T, Peters CJ, Rollin PE, Ksiazek TG, Nichol ST. Genetic diversity and distribution of Peromyscus-borne hantaviruses in North America. Emerg Infect Dis 1999;5:75-86.
7. Caire W, Tyler JD, Glass BP, Mares MA. Mammals of Oklahoma. Norman (OK): University of Oklahoma Press; 1989. 567 p.
8. Murray NL, editor. Oklahoma's bio-diversity plan: a shared vision for conserving our natural history. Oklahoma City: Oklahoma Department of Wildlife Conservation; 1996. 129 p.
9. Mills JN, Childs JE. Ecologic studies of rodent reservoirs: their relevance for human health. Emerg Infect Dis 1998; 4:529-537.
10. (Anonymous) American Society of Mammalogists guidelines, Acceptable field methods in mammalogy: preliminary guidelines approved by the American Society of Mammalogists. J Mammal 1987; Suppl to Vol 68:1-18.
11. Centers for Disease Control and Prevention [CDC], Hantavirus infection—Southwestern United States: interim recommendations for risk reduction. MMWR (July 30) 1993; 42, RR-11:i-13.
12. Orlans FB, editor. Field Research Guidelines. Bethesda (MD): Scientists Center for Animal Welfare; 1988. 23.
13. Swann DE, Kuenzi AJ, Morrison ML, DeStefano S. Effects of sampling blood on survival of small mammals. J Mammal 1997;78:908-913.
14. Fulhorst CF, Bowen MD, Ksiazek TG, Rollin PE, Nichol ST, Kosoy MY, Peters CJ. Isolation and characterization of Whitewater Arroyo virus, a novel
{Page 65}
North American arenavirus. Virology 1996;224:114-120.
15. Drickamer LC, Feldhamer GA, Mikesic DG, Holmes CM. Trap-response heterogeneity of house mice (Mus musculus) in outdoor enclosures. J Mammal 1999;80:410-420.
16. Lewellen RH, Vessey SH. Estimating densities of Peromyscus leucopus using live-trap and nestbox censuses. J Mammal 1999;80:400-409.
17. Bennett SG, Webb JP Jr., Madon MB, Childs JE, Ksiazek TG, Torrez-Martinez N, Hjelle B. Hantavirus (Bunyaviridae) infections in rodents from Orange and San Diego Counties, California. Am J Trop Med Hyg 1999;60:75-84.
18. Otteson EW, Riolo J, Rowe JE, Nichol ST, Ksiazek TG, Rollin PE, St. Jeor SC. Occurrence of hantavirus within the rodent population of Northeastern California and Nevada. Am J Trop Med Hyg 1996;54:127-133.
19. Jay M, Ascher MS, Chomel BB, Madon M, Sesline D, Enge BA, Hjelle B, Ksiazek TG, Rollin PE, Kass PH, Reilly K. Seroepidemiological studies of hantavirus infection among wild rodents in California. Emerg Infect Dis 1997;3:183-190.
20. Hjelle B, Chavez-Giles F, Torrez-Martinez N, Yates T, Sarisky J, Webb J, Ascher M. Genetic identification of a novel hantavirus of the harvest mouse, Reithrodontomys megalotis. J Virol 1994;68:6751-6754.
21. Abbott KD, Ksiazek TG, Mills JN. Long-term hantavirus persistence in rodent populations in Central Arizona. Emerg Infect Dis 1999;5:102-112.
22. Calisher CH, Sweeney W, Mills JN, Beaty BJ, Natural history of Sin Nombre virus in western Colorado. Emerg Infect Dis 1999;5:126-134.
23. Kuenzi AJ, Morrison ML, Swann DE, Hardy PC, Downward GT. A longitudinal study of Sin Nombre Virus prevalence in rodents, Southeastern Arizona. Emerg Infect Dis 1999; 5:113-117.
24. Ksiazek TG, Nichol ST, Mills JN, Groves MG, Wozniak A, McAdams S, Monroe MC, Johnson AM, Martin ML, Peters CJ, Rollin, PE. Isolation, genetic diversity, and geographic distribution of Bayou Virus (Bunyaviridae: Hantavi-rus). Am J Trop Med Hyg 1997;57:445-448.
25. Torrez-Martinez N, Bharadwaj M, Goade D, Delury J, Moran P, Hicks B, Nix B, DaviesJL, Hjelle B. Bayou Virus-associated hantavirus pulmonary syndrome in eastern Texas: Identification of the rice rat, Oryzomys palustris, as reservoir host. Emerg Infect Dis 1998;4: 105-111.
26. Boone JD, Otteson EW, McGwire KC, Villard P, Rowe JE, St. Jeor SC. Ecology and demographics of hantavirus infections in rodent populations in the Walker River Basin of Nevada and California. Am J Trop Med Hyg 1998; 59:445-451.
27. M'Closkey RT. Community structure in sympatric rodents. Ecology 1976; 57:729-739.
28. Hjelle B, Lee S-W, Song W, Torrez-Martinez N, Song J-W, Yanagihara R, Gavrilovskaya I, Machow ER. Molecular linkage of hantavirus pulmonary syndrome to the white-footed mouse, Peromyscus leucopus: genetic characterization of the M genome of New York virus. J Virol 1995;69:8137-8141.
29. Rhodes LV III, Huang C, Sanchez AJ, Nichol ST, Zaki SR, Ksiazek TG, Humphreys JG, Freeman JJ, Knect KR. Hantavirus pulmonary syndrome associated with Monongahela virus, Pennsylvania. Emerg Infect Dis 2000;6:616-621.
30. Weigler BJ, Ksiazek TG, Vandenbergh JG, Lewin M, Sullivan WT. Serological evidence for zoonotic hantaviruses in North Carolina rodents. J Wildl Dis 1996;32:354-357.
31. Glass GE, Livingstone W, Mills JN, Hlady WG, Fine JB, Biggler W, Coke T, Frazier D, Atherley S, Rollin PR, Ksiazek TG, Peters CJ, Childs JE. Black Creek Canal virus infection in Sigmodon hispidus in Southern Florida. Am J Trop Med Hyg 1998;59:699-703.
32. Graham TB, Chomel BB. Population dynamics of the deer mouse (Pero-myscus maniculatus) and Sin Nombre Virus, California Channel Islands. Emerg Infect Dis 1997;3:367-370.
{Page 66}
33. Mills JN, Ksiazek TG, Peters CJ, Childs JE. Long-term studies of hantavirus reservoir populations in the Southwestern United States: a synthesis. Emerg Infect Dis 1999;5:135-142.
34. Glass GE, Childs JE, Korch GW, LeDuc JW. Association of intraspecific wounding with hantaviral infection in wild rats (Rattas norvegicus). Epidemiol Infect 1988;101:459-472.
35. Schmidt CA. Variation and congruence of microsatellite markers for Peromyscus leucopus, J Mammal 1999;80:522-529.
36. Morzunov SP, Rowe JE, Ksiazek TG, Peters CJ, St. Jeor SC, Nichol ST. Genetic analysis of the diversity and origin of hantaviruses in Peromyscus leucopus mice in North America. J Virol 1998; 72:57-64.
37. Enserink M. Emerging diseases: New arenavirus blamed for recent deaths in California. Science 2000;289(5481):842-843.
38. Centers for Disease Control and Prevention. Fatal illnesses associated with a New World arenavirus—California, 1999-2000. MMWR 2000;49:709-711.
39. Calisher CH, Nabity S, Root JJ, Fulhorst CF, Beaty BJ. Transmission of an arena-virus in white-throated woodrats (Neotoma albigula), Southeastern Colorado, 1995-1999. Emerg Infect Dis 2001;7:397-402.
40. Fulhorst CF, Charrel RN, Weaver SC, Ksiazek TG, Bradley RD, Milazzo ML, Tesh RB, Bowen MD. Geographic distribution and genetic diversity of Whitewater Arroyo Virus in the Southwestern United States. Emerg Infect Dis 2001;7:403-407.
41. Kaufman GA, Kaufman DW, McMillan BR, Brillhart DE. Prevalence of hanta-virus antibodies in natural populations of deer mice in north central Kansas. Prairie Nat 1994; 26:209-216.
42. Rawlings JA, Torrez-Martinez N, Neill SU, Moore GM, Hicks BN, Pichuantes S, Nguyen A, Bharadwaj M, Hjelle B. Cocirculation of multiple hantaviruses in Texas, with characterization of the small (S) genome of a previously undescribed virus of cotton rats (Sigmo-don hispidus). Am J Trop Med Hyg 1996;55:72-79.
43. Drebot MA, Artsob H. Identification of a putative recombinant hantavirus displaying nucleotide and amino acid sequence homologies to both Sin Nombre and Prospect Hill viruses. Emergence and control of rodent-borne viral diseases (Hantaviruses and Arenaviruses), program 1998. Lyon (France): Fonda-tion Marcel Mérieux, p. 119.
44. Mackelprang R, Dearing MD, St. Jeor S. High prevalence of Sin Nombre Virus in rodent populations, Central Utah: A consequence of human disturbance? Emerg Infect Dis 2001; 7:480-482.
45. Langlois JP, Fahrig L, Merriam G, Artsob H. Landscape structure influences continental distribution of hanta-virus in deer mice. Landscape Ecol 2001;16:255-266.
46. Houck MA, Qin H, Roberts HR. Hanta-virus transmission: Potential role of ectoparasites, Vector-Borne & Zoonotic Diseases 2001;1:75-79.
47. Engelthaler DM, Mosley DG, Cheek JE, Levy CE, Komatsu KK, Ettestad P, Davis T, Tanda DT, Miller L, Frampton JW, Porter R, Bryan RT. Climatic and environmental patterns associated with hantavirus pulmonary syndrome, Four Corners Region, United States. Emerg Infect Dis 1999;5:87-94.
48. Nisbett NL. Knowledge and health beliefs concerning hantavirus among pest control technicians in Oklahoma [MS thesis]. Oklahoma City: University of Oklahoma Health Sciences Center; 2000. 63 p. Available from: OUHSC Library.
49. Centers for Disease Control and Prevention. Hantavirus pulmonary syndrome—Vermont, 2000. MMWR 2001; 50:603-605.
50. Hantavirus in the Americas: guidelines for prevention, diagnosis, treatment and control. Washington DC: Pan American Health Organization, Technical Paper No. 47; 2000. 63 p.
Received: March 14, 2001; Accepted: May 12, 2001